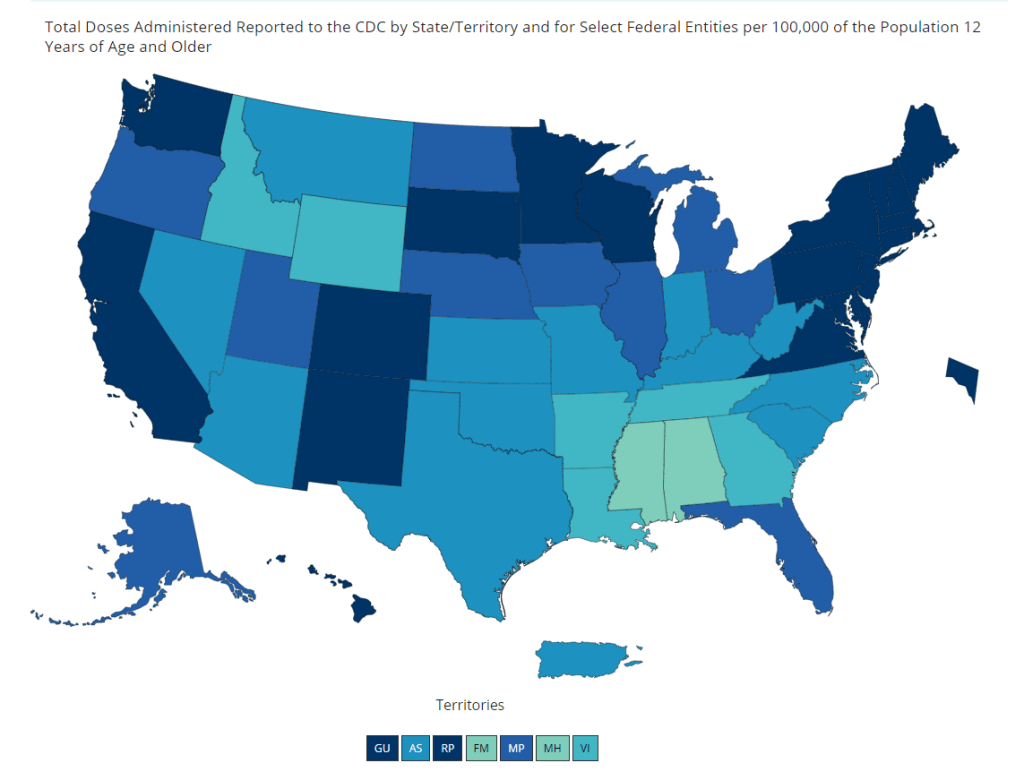
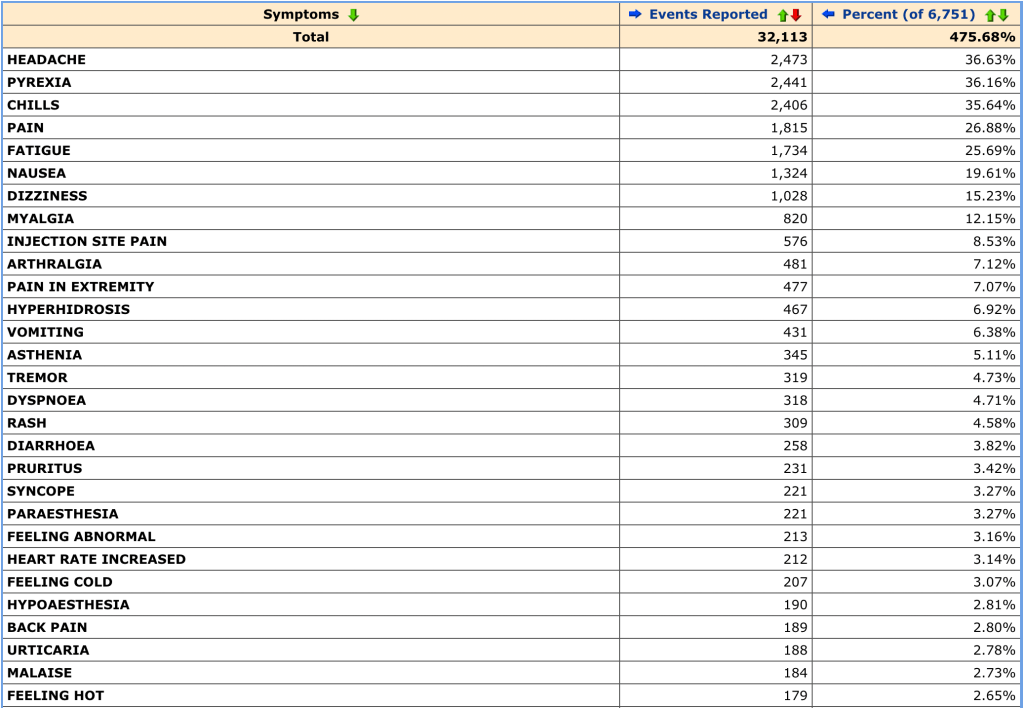
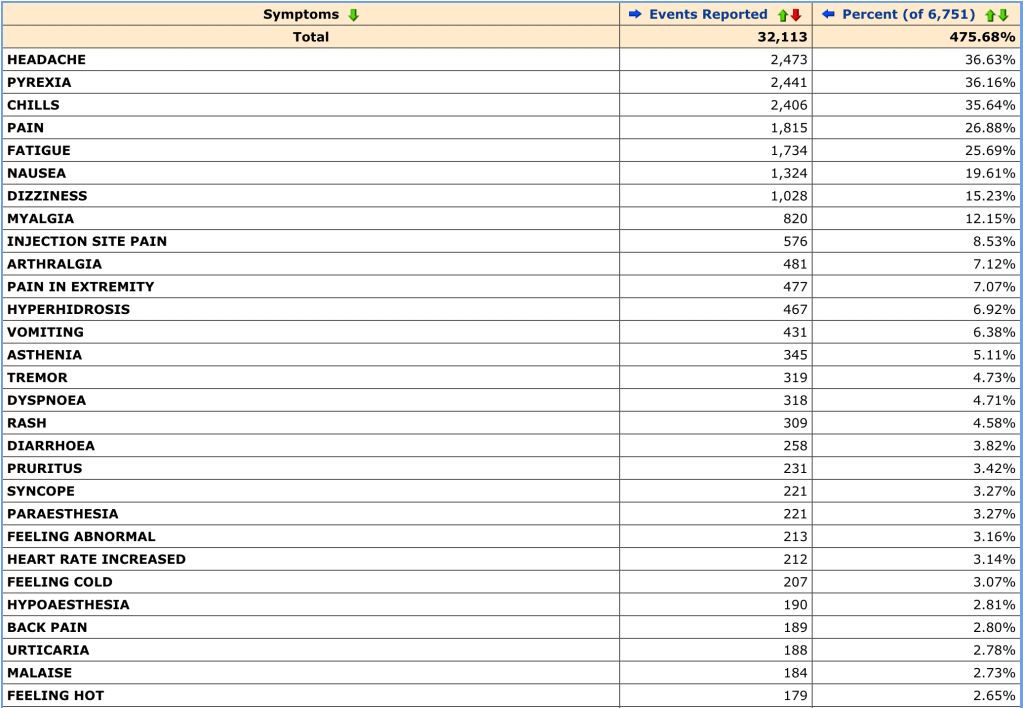
Some good global vaccine news this week: it looks like vaccine cocktails may be a promising option.
A clinical trial based in Spain of around 600 participants (aged 18-59) reported encouraging results regarding mix-and-match vaccines (or “heterologous prime-and-boost,” if you want the jargon) meaning one shot of one vaccine and the second shot of another. In this study, the first dose given was AstraZeneca, and the second was Pfizer.
The study found that protective IgG antibodies were 30-40 times higher in the treatment group than the control group (those who had only received the first dose of the AstraZeneca vaccine). Neutralizing antibodies were also seven times higher after the Pfizer dose compared to the control, while usually they double in number after the second AstraZeneca shot.
As some people familiar with Covid vaccines may note, these vaccines use two different mechanisms to stimulate the immune system: the AstraZeneca shot uses an adenovirus vector modified with the SARS-CoV-2 spike protein while the Pfizer vaccine uses messenger RNA to coax cells into making the spike protein themselves. This early success demonstrates that vaccines with different mechanisms can be combined to induce a strong immune response.
In the wake of the AstraZeneca blood clot news, it’s reasonable to expect that some may be hesitant to get the second shot if they have received the first AstraZeneca shot. Some authorities have advised people who have gotten the first dose of AstraZeneca to get an alternative for the second shot. Having an alternative that hasn’t been linked to blood clots might persuade those hesitant to get the second AstraZeneca shot to complete a vaccination regimen, especially if it might stimulate even more of an immune response than the regular AstraZeneca regimen.
There’s currently another heterologous prime-and-boost trial in place in the United Kingdom with a slightly more complicated experimental setup (the four groups were AstraZeneca for both shots, Pfizer for both shots, Pfizer for the first and AstraZeneca for the second, or vice versa), with all participants over 50.
This study hasn’t reported results regarding immune responses yet, but they have reported some preliminary reactogenicity results. On May 12, researchers reported that mild side effects like fever or fatigue were more common in people who had received mixed vaccines. However, there were no severe side effects, and the mild ones subsided after a few days. The Spanish study did not find this, and instead found that mild side effects were about as common as they were with a regular vaccine regimen.
The UK study is expected to report immune response data soon, so it’ll be interesting to see if it matches the results found by the Spanish study. We’ll keep you updated when those results come out.
More vaccine reporting
- Sources and updates, November 12Sources and updates for the week of November 12 include new vaccination data, a rapid test receiving FDA approval, treatment guidelines, and more.
- How is the CDC tracking the latest round of COVID-19 vaccines?Following the end of the federal public health emergency in May, the CDC has lost its authority to collect vaccination data from all state and local health agencies that keep immunization records. As a result, the CDC is no longer providing comprehensive vaccination numbers on its COVID-19 dashboards. But we still have some information about this year’s vaccination campaign, thanks to continued CDC efforts as well as reporting by other health agencies and research organizations.
- Sources and updates, October 8Sources and updates for the week of October 8 include new papers about booster shot uptake, at-home tests, and Long COVID symptoms.
- COVID source shout-out: Novavax’s booster is now availableThis week, the FDA authorized Novavax’s updated COVID-19 vaccine. Here’s why some people are excited to get Novavax’s vaccine this fall, as opposed to Pfizer’s or Moderna’s.
- COVID-19 vaccine issues: Stories from COVID-19 Data Dispatch readers across the U.S.Last week, I asked you, COVID-19 Data Dispatch readers, to send me your stories of challenges you experienced when trying to get this fall’s COVID-19 vaccines. I received 35 responses from readers across the country, demonstrating issues with insurance coverage, pharmacy logistics, and more.