Last week, I asked readers to fill out a survey designed to help me reflect on the COVID-19 Data Dispatch’s future. Though the Delta surge—and the pandemic as a whole—is far from over, I’m considering how this publication may evolve in a “post-COVID” era. Specifically, I’m thinking about how to continue serving readers and other journalists as we prepare for future public health crises.
Thank you to everyone who’s filled out the survey so far! I really appreciate all of your feedback. If you haven’t filled it out yet, you can do so here.
Besides some broader questions about the CDD’s format and topics we may explore in the future, the survey asked readers to submit questions that they have about COVID-19 in the U.S. right now. In the absence of other major headlines this week, I’m devoting this week’s issue to answering a few of those questions.
Should I get a booster shot? If so, should it be a different one from the first vaccine I got? When will my kids (5-11) likely be eligible?
I am not a doctor, and I’m definitely not qualified to give medical advice. So, the main thing I will say here is: identify a doctor that you trust, and talk to them about booster shots. I understand that a lot of Americans don’t have a primary care provider or other ways to easily access medical advice, though, so I will offer some more thoughts here.
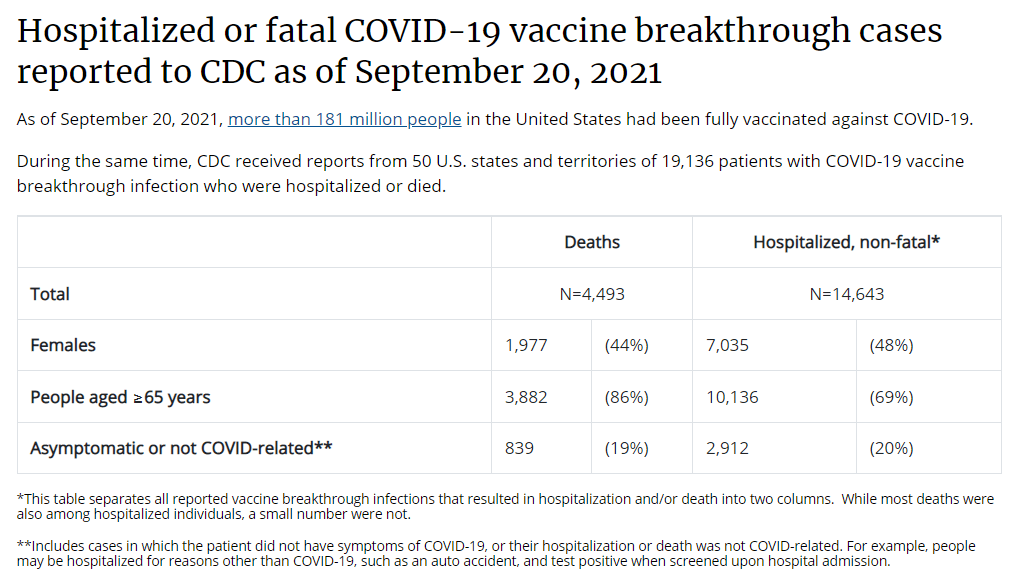
As I wrote last week, we do not have a lot of data on who’s most vulnerable to breakthrough COVID-19 cases. We do know that seniors are more vulnerable—this is one point where most experts agree. We know that adults with the same health conditions that make them more likely to have a severe COVID-19 case without a vaccine (autoimmune conditions, diabetes, kidney disease, etc.) are also more vulnerable to breakthrough cases, though we don’t have as much data here. And we know that vaccinated adults working in higher-risk locations like hospitals, nursing homes, and prisons are more likely to encounter the coronavirus, even if they may not necessarily be more likely to have a severe breakthrough case.
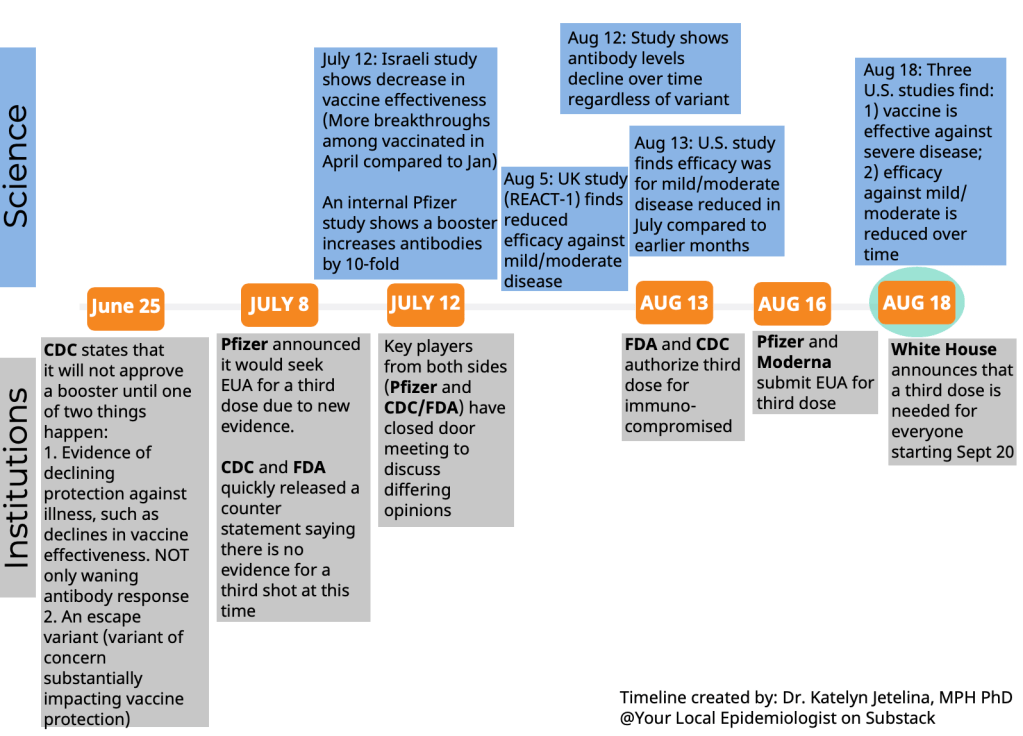
The FDA and CDC’s booster shot guidance is intentionally broad, allowing many Americans to receive a booster even if it is not necessarily needed. So, consider: what benefits would a booster shot bring you? Are you a senior or someone with a health condition that makes you more likely to have a severe COVID-19 case? Do you want to protect the people you work or live with from potentially encountering the coronavirus?
If you answered “yes” to one of those questions, a booster shot may make sense for you. And, while you may be angry about global vaccine inequity, one individual refusal of a booster shot would not have a significant impact on the situation. Rather, many vaccine doses in the U.S. may go to waste if not used for boosters. But again: talk to your doctor, if you’re able to, about this decision.
Currently, Pfizer booster shots are available for people who previously got vaccinated with Pfizer. The FDA’s vaccine advisory committee is meeting soon to discuss Moderna and Johnson & Johnson boosters: they’ll discuss Moderna on October 14 and J&J on October 15. Vaccine approval in the U.S. depends upon data submission from vaccine manufacturers—and vaccine manufacturers have not been studying mix-and-match booster regimens—so coming approvals will likely require Americans to get a booster of the same vaccine that they received initially. We will likely see more discussion of mix-and-match vaccinations in the future, though, as more outside studies are completed.
As for when your kids will likely be eligible: FDA’s advisory committee is meeting to discuss Pfizer shots for kids ages 5 through 11 on October 26. If that meeting—and a subsequent CDC meeting—goes well, kids may be able to get vaccinated within a week of that meeting. (Potentially even on Halloween!)
Why don’t people get vaccinated and how can we make them?
I got a couple of questions along these lines, asking about vaccination motivations. To answer, I’m turning to KFF’s COVID-19 Vaccine Monitor, a source of survey data on vaccination that I (and many other journalists) have relied on since early 2021.
KFF released the latest round of data from its vaccine monitor this week. Here are a few key takeaways:
- The racial gap in vaccinations appears to be closing. KFF found that 71% of white adults have been vaccinated, compared to 70% of Black adults and 73% of Hispanic adults. Data from the CDC and Bloomberg (compiling data from states) similarly show this gap closing, though some parts of the country are more equitably vaccinated than others.
- A massive partisan gap in vaccinations remains. According to KFF, 90% of Democrats are vaccinated compared to just 58% of Republicans. This demonstrates the pervasiveness of anti-vaccine misinformation and political rhetoric among conservatives.
- Rural and younger uninsured Americans also have low vaccination rates (62% and 54%, respectively). Both rural and uninsured people have been neglected by the U.S. healthcare system and face access barriers; for more on this topic, I recommend this Undark article by Timothy Delizza.
- Delta was a big vaccination motivator. KFF specifically asked people who had gotten their shots after June 1 why they chose to get vaccinated. The most popular reasons were, in order: the increase in cases due to Delta (39%), concern about reports of local hospitals and ICUs filling with COVID-19 patients (38%), and knowing someone who got seriously ill or died from COVID-19 (36%).
- Mandates and social pressures were also vaccination motivators. 35% of KFF’s recently vaccinated survey respondents said that a big reason for their choice was a desire to participate in activities that require vaccination, like going to the gym, a big event, or traveling. 19% cited an employer requirement and 19% cited social pressure from family and friends.
The second part of this question, “how can we make them?”, reflects a dangerous attitude that has permeated vaccine conversations in recent months. Yes, it’s understandable to be frustrated with the Americans who have refused vaccination. But we can’t “make” the unvaccinated do anything, and such a forceful attitude may put off people who still have questions about the vaccines or who have faced discrimination in the healthcare system. To increase vaccinations among people who are still hesitant, it’s important to remain open-minded, not condescending. For more: read Ed Yong’s interview with Dr. Rhea Boyd.
That said, we’re now getting a sense of which strategies can increase vaccination: employer mandates, vaccination requirements for public life, and personal experience with the coronavirus. As the Delta surge wanes, it will take more vaccination requirements and careful, open-minded conversations to continue motivating people to get their shots.
What are some things I might say to convince people of Delta’s severity and the need to not relax on masking, distancing, etc?
To answer this, I’ll refer you to the article I wrote about Delta on August 1, as the findings that I discuss there have been backed up by further research.
Personally, there are two statistics that I use to express Delta’s dangers to people:
- Delta causes a viral load 1,000 times higher than the original coronavirus strain. This number comes from a study in Guangzhou, China, posted as a preprint in late July. While viral load does not correspond precisely to infectiousness (there are other viral and immune system factors at play), I find that this “1,000 times higher” statistic is a good way to convey just how contagious Delta is, compared to past variants.
- An interaction of one second is enough time for Delta to spread from one person to another. Remember the 15-minute rule? In spring 2020, being indoors with someone, unmasked, for 15 minutes or more was considered “close contact.” Delta’s increased transmissibility means that an interaction of one second is now enough to be a “close contact.” The risk is lower if you’re vaccinated, but still—Delta is capable of spreading very quickly in enclosed spaces.
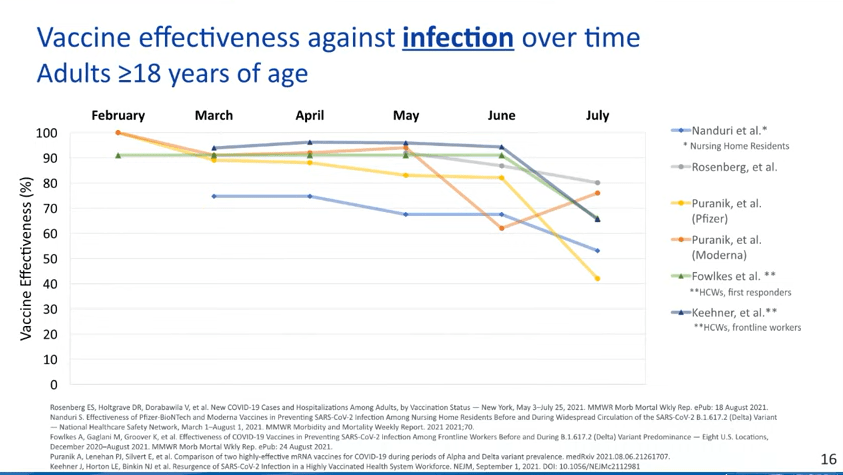
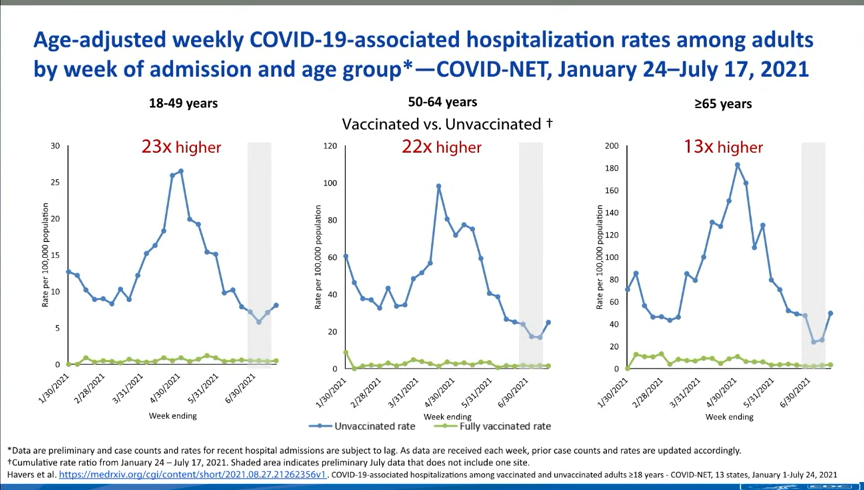
You may also find it helpful to discuss rising numbers of breakthrough cases in the U.S. While vaccinated people continue to be incredibly well protected against severe disease and death caused by Delta, the vaccines are not as protective against coronavirus infection and transmission. (They are protective to some degree, though! Notably, coronavirus infections in vaccinated people tend to be significantly shorter than they are in the unvaccinated, since immune systems can quickly respond to the threat.)
It’s true that rising breakthrough case numbers are, in a way, expected—as more people get vaccinated, breakthrough cases will naturally become more common, because the virus has fewer and fewer unvaccinated people to infect. But considering the risks of spreading the coronavirus to others, plus the risks of Long COVID from a breakthrough case… I personally don’t want a breakthrough case, and so I continue masking up and following other safety protocols.
What monitoring do we have in place for COVID “longhaulers” and their symptoms/health implications?
This is a great question, and one I wish I could answer in more detail. Unlike COVID-19 cases, hospitalizations, and other major metrics, we do not have a comprehensive national monitoring system to tell us how many people are facing long-term symptoms from a coronavirus infection, much less how they’re faring. I consider this one of the country’s biggest COVID-19 data gaps, leaving us relatively unprepared to help the thousands, if not millions, of people left newly disabled by the pandemic.
In February, the National Institutes of Health (NIH) announced a major research initiative to study Long COVID. Congress has provided over $1 billion in funding for the research. This initiative will likely be our best source for Long COVID information in the future, but it’s still in early stages right now. Just two weeks ago, the NIH awarded a large share of its funding to New York University’s Langone Medical Center; NYU is now setting up long-term studies and distributing funding to other research institutions.
As I wrote in the September 19 issue, the NIH’s RECOVER website currently reports that between 10% and 30% of people infected with the coronavirus will go on to develop Long COVID; hopefully research at NYU and elsewhere will lead to some more precise numbers.
While we wait for the NIH research to progress, I personally find the Patient-Led Research Collaborative (PLRC) to be a great source for Long COVID research and data. The PLRC consists of Long COVID patients who research their own condition; it was founded out of Body Politic’s Long COVID support group. This group produced one of the most comprehensive papers on Long COVID to date, based on an international survey including thousands of patients, and has more research currently ongoing.
If you have the means to support Long COVID patients—many of whom are unable to work and facing homelessness—please see the responses to this tweet by PLRC researcher Hannah Davis:
Why is the CDC not doing comprehensive high volumes of sequencing on all breakthrough cases at the very least?
I wish I knew! As I wrote last week (and in several other past issues), the lack of comprehensive breakthrough case data in the U.S. has contributed to a lack of clarity on booster shots, as well as a lack of preparedness for the next variants that may become threats after Delta. The CDC’s inability to track and sequence all breakthrough cases—not just the severe ones—is dangerous.
That said, it is very difficult to track breakthrough cases in a country like the U.S. Consider: the U.S. does not have a comprehensive, national electronic records system for patients admitted to hospitals, much less those who receive COVID-19 tests and other care at outpatient clinics. This lack of comprehensive records makes it difficult to match people who’ve been vaccinated with those who have received a positive COVID-19 test. Thousands, if not millions of Americans are now relying on rapid tests for their personal COVID-19 information—and most rapid tests don’t get entered into the public health records system at all.
Plus, local public health departments are chronically underfunded, understaffed, and burned out after almost two years of working in a pandemic; they have little bandwidth to track breakthrough cases. Many Americans refuse to participate in contact tracing, which hinders the public health system’s ability to collect key information about their cases. And there are other logistical challenges around genomic sequencing; despite new investments in this area, many parts of the country don’t have sequencing capacity, or the information infrastructure needed to send sequencing results to the CDC.
So, if the CDC were tracking non-severe breakthrough cases, they’d likely miss a lot of the cases. But that doesn’t mean they shouldn’t be trying, in my opinion.
How safe is it to visit my family for the holidays?
This is another place where I don’t feel qualified to give advice, but I can offer some thoughts. If I were you, I would think about the different ways in which holiday travel might pose risk to me and to the people at the other end of my trip. I would consider:
- Quarantining beforehand. Do your occupation and living circumstances allow you to quarantine for a week, or at least limit your exposure to settings where you might be at risk of catching the coronavirus, before you travel? Can you get a test before traveling?
- Types of travel. Can you make the trip in a car or on public transportation, or do you need to fly? If you need to fly, can you select an airline that has stricter COVID-19 safety requirements? (United recently reported that over 96% of its employees are now vaccinated, for example.) Can you wear a high-quality mask for the flight?
- Quarantining and/or testing upon arrival. Can you spend a couple of days in quarantine once you get to your destination? Would you have access to testing (with results in under 24 hours) upon your arrival, or would you be able to bring rapid tests with you?
- Who you’re spending time with. Among the family you’d be visiting, is everyone vaccinated (besides young children)? If anyone is not vaccinated, could your potential travel be a motivator to help convince them to get vaccinated? Does the group include seniors or people with health conditions that put them at high risk for COVID-19, and if so, can they get booster shots?
- Activities that you do at your destination. Would you be able to have large gatherings outside, or in a well-ventilated space? What else can you do to reduce the risk of these activities?
Like other activities, travel can be relatively safe or fairly dangerous depending on the precautions that you’re able to take, and depending on COVID-19 case rates where you live and at your destination. And, like other activities, your choice to travel or not travel depends a lot on your personal risk tolerance. Nothing is zero-risk right now; each person has a threshold that determines what level of COVID-19 risk they are and are not comfortable taking. Through some self-reflection, you can determine if travel is above or below your risk threshold.
Why are policies so different now than they were at this time last year?
Public health tends to go through cycles of “panic” and “neglect.” Ed Yong’s latest feature goes into the history of this phenomenon:
Almost 20 years ago, the historians of medicine Elizabeth Fee and Theodore Brown lamented that the U.S. had “failed to sustain progress in any coherent manner” in its capacity to handle infectious diseases. With every new pathogen—cholera in the 1830s, HIV in the 1980s—Americans rediscover the weaknesses in the country’s health system, briefly attempt to address the problem, and then “let our interest lapse when the immediate crisis seems to be over,” Fee and Brown wrote. The result is a Sisyphean cycle of panic and neglect that is now spinning in its third century. Progress is always undone; promise, always unfulfilled. Fee died in 2018, two years before SARS-CoV-2 arose. But in documenting America’s past, she foresaw its pandemic present—and its likely future.
During the COVID-19 pandemic, the U.S. took a nosedive into the “neglect” cycle before we were even finished with the “panic” cycle. Congress has already slashed its funding for future pandemic preparedness, while state and local governments across the country restrict the powers of public health officials. As a result, we’re seeing an “everyone for themselves” attitude at a time when we should be seeing new mask mandates, restrictions on public activities, and other safety measures.
Basically, America decided the pandemic was over and acted accordingly—and if you get COVID-19 now, it’s “your fault for not being vaccinated.” This phenomenon has been especially pronounced in rural areas, which struggled a lot (but saw few cases) during spring 2020 lockdowns and are extremely hesitant to do anything approaching a “lockdown” again.
We need an attitude shift—and more investment in public health—to actually end this pandemic and prepare for the next health crisis. Yong’s feature goes into this in more detail; definitely give that a read if you haven’t yet.
When is this going to be over?!?
Unfortunately, this is very hard to predict—even for the expert epidemiologists and computational biologists who make the models. Check out the CDC’s compilation of COVID-19 case models: most of them agree that cases will keep going down in the coming weeks, but they’re kind of all over the place.
Last week, I summarized two stories—from The Atlantic and STAT News—that discuss the coming winter, and kind of get at this question. It’s possible that cases keep declining from their present numbers, and that the Delta surge we just faced is the last major surge in the U.S. It’s also possible that a new variant arises out of Delta and sends us into yet another new surge. If that happens, more people will be protected by vaccination and prior infection, but healthcare systems could come under strain once again.
As long as the coronavirus continues spreading somewhere in the world, it will continue to pose risk to everyone—able to cause new outbreaks and mutate into new variants. This will continue until the vast majority of the world is vaccinated. And then, at some point, the coronavirus will probably become endemic, meaning it persists in the population at some kind of “acceptable” threshold. Just like the flu.
Dr. Ellie Murray, epidemiologist at Boston University’s School of Public Health, explained how a pandemic becomes endemic in a recent Twitter thread:
Dr. Murray points out that, even when a disease reaches endemic status, tons of scientists and public health workers will still continue to monitor it. This is the case for the flu—think about all of the effort that goes into a given year’s flu shot!—and it will likely be the case for COVID-19.
In short, public health leaders need to figure out what level of COVID-19 transmission is “acceptable” and how we will continue to monitor it. This needs to happen at both U.S. and global levels. And, thanks to our vaccine-rich status, it’ll likely happen in the U.S. long before it happens globally.
Again, if you haven’t filled out the survey yet, you can do so here. I may answer more questions next week!