- Grants to help with global pandemic preparedness: This week, the World Bank’s Pandemic Fund announced the recipients of its first round of grants. The fund is a finance initiative to “strengthen pandemic prevention, preparedness, and response capacities,” particularly for low- and middle-income countries. Its first round of grants will go to 37 countries across 6 global regions, distributing $338 million in funding. The full list of awards is available on the World Bank’s website.
- Genetic marker of asymptomatic COVID-19: A new paper published in Nature this week reports on a common genetic marker that may lead people to have symptom-free COVID-19 cases. The researchers (a team from the University of California San Francisco and other institutions) searched for genetic patterns among 30,000 people who shared their COVID-19 symptom information through a smartphone app. They found a correlation between asymptomatic infection and a specific version of a gene related to T cells. As Eric Topol notes in his newsletter, this study follows two others that examined genetic markers of Long COVID.
- Quantifying cognitive symptoms of Long COVID: Speaking of Long COVID: researchers at Kings College London studied the condition’s cognitive symptoms (also called brain fog) by measuring patients’ performance in different mental tasks. The study included over 3,000 participants, more than half of whom completed two rounds of testing over two years. Overall, the researchers found that cognitive symptoms persisted for nearly two years after patients’ initial infections, and most severe for patients with the longest-lasting Long COVID impacts. For these patients, “the effect of COVID-19 on test accuracy was comparable in size to the effect of a 10-year increase in age,” per a press release by Kings College London.
- Long COVID is common in children: Another Long COVID study published this week: researchers at a hospital in Toronto compiled a review paper examining the condition’s prevalence among children. Their review included 30 studies including about 15,000 total pediatric patients. Across all the studies, researchers reported that about 16% of children experienced at least one Long COVID symptom three or more months after their COVID-19 infections. However, compiling these data was a challenging task because different studies used different definitions of Long COVID, different methods of following up with patients over time, and other inconsistencies, the authors wrote.
- Dogs detecting COVID-19 through scent: One more paper that stuck out to me this week: a pair of researchers (one at the University of California, Santa Barbara and one at a biotech company focused on sniffing for COVID-19) examined how well dogs can detect the coronavirus. This was also a review paper, including 29 studies and 31,000 COVID-19 test samples. Overall, the dogs performed with similar accuracy to PCR tests, researchers found. “We believe that scent dogs deserve their place as a serious diagnostic methodology that could be particularly useful during pandemics,” one of the authors said in a statement.
- Monoclonal antibody to protect babies from RSV: Finally, a bit of good news for combatting another common respiratory virus: the FDA has approved a new monoclonal antibody treatment to protect infants and young children against RSV. The therapy is likely to be recommended by the CDC and manufactured in time for respiratory virus season this fall. In clinical trials, it lowered the risk of an RSV infection requiring medical care by about 76%—which is a big deal for a disease that leads to more babies in hospitals than any other in the U.S., reports Helen Branswell at STAT.
Tag: Long COVID
-
Sources and updates, July 23
-
Sources and updates, July 16
- Real-time detection of coronavirus in the air: A new study, published this week in Nature Communications, describes a tool to detect airborne SARS-CoV-2 particles. Researchers at Washington University in St. Louis developed this tool; it works by collecting aerosols in a container and screening them for chemical properties matching the coronavirus spike protein. In the researcher’s proof-of-concept study, the detector tool was able to detect coronavirus particles with 77% to 83% accuracy, and could detect the virus when it was present at relatively small volumes. If the tool holds up to further tests, it could be valuable for monitoring healthcare settings and other public places.
- Routine respiratory virus testing at K-12 schools: Another study about testing, published in the CDC’s Morbidity and Mortality Weekly Report: researchers in Kansas City, Missouri regularly tested students and staff members at the public school district for SARS-CoV-2, the flu, RSV, and several other common respiratory viruses. About 900 participants opted into monthly testing for the 2022-23 school year, for a total of 3,200 tests conducted. Overall, about one in four tests were positive for at least one respiratory virus. Pre-K students had the highest positivity rate (40%), while rhinovirus/enterovirus was most commonly detected. The study shows how many viruses are going around in school settings, as well as the potential value of testing for reducing spread.
- Predicting COVID-19 activity with Google searches: COVID-19 data commentators have long suspected that online trends indicating people were losing their sense of smell or taste in large numbers could predict an upcoming surge. (Remember the Yankee Candle Index?) Well, a new study in the CDC’s Emerging Infectious Diseases journal provides some evidence for this pattern. Researchers at Yale and Columbia Universities compared Google search trends for “loss of smell” and “loss of taste” to COVID-19 hospitalization and death numbers in five countries. They found a strong correlation between these searches and COVID-19 increases for major COVID-19 waves. So, even as official data become less available, online trends may still be a good indicator.
- Estimating infection rates from mortality data: COVID-19 mortality data can be used to work backward and estimate true infection rates, according to a new paper in Science by researchers at the University of California Davis and the University of the Basque Country (in Spain). The scientists used a machine learning model to analyze death reports from several European countries, essentially predicting infection rates in reverse. Their analysis found that lockdowns and mask requirements, among other COVID-19 safety measures, had a major impact on transmission, one of the authors said in a press release. Mortality data continues to present a useful tool for tracking COVID-19’s full impact.
- Long COVID cohort study suggests full recovery may be rare: One more notable new study, shared by The Lancet as a preprint: researchers at a hospital in Barcelona shared the results of a study following Long COVID patients for two years. The study followed 548 people, including 341 with Long COVID and 207 who did not have long-term symptoms after acute COVID-19. Only 26 (7.6%) of the Long COVID patients recovered during the two-year follow-up period, according to symptom surveys and diagnostic testing. Hannah Davis, a patient-researcher at the Patient-Led Research Collaborative, shared additional highlights and takeaways from the study in a Twitter thread.
- New bill to strengthen wastewater surveillance: Finally, a bit of hopeful news: three U.S. senators just introduced a bipartisan bill that would strengthen the CDC’s National Wastewater Surveillance System (NWSS). The bill would specifically expand NWSS to include surveillance for other public health threats, and would enable it to provide more funding to state and local health agencies. Cory Booker from New Jersey, Angus King from Maine, and Mitt Romney from Utah are the three sponsors. I’m not a political reporter, so I won’t pretend to know how likely this bill’s chances are of passing, but I hope it’s a step toward making the U.S.’s wastewater surveillance infrastructure permanent.
Editor’s note, July 23, 2023: An earlier version of this post misstated the virus most commonly detected in the Kansas City schools study. (It was rhinovirus/enterovirus, not RSV.)
-
Sources and updates, June 11
- Quantifying Long COVID’s impact on day-to-day life: A new study published this week in the BMJ is one of the first I’ve seen to focus not on Long COVID’s symptoms, but on how it impacts quality of life for patients. Researchers at University College London assessed life impacts for about 3,700 Long COVID patients using surveys in an online health platform. The surveys found that “Long COVID can leave people with worse fatigue and quality of life than some cancers, yet the support and understanding is not at the same level,” study coauthor Dr. William Henley said in a statement about the research. This study confirms what I’ve heard from many long-haulers in interviews over the last couple of years.
- Long COVID and ME/CFS similarities: Another notable Long COVID paper: two leading experts on chronic illness, Dr. Anthony Komaroff at Harvard Medical School and W. Ian Lipkin at Columbia University, wrote a detailed review identifying commonalities between Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a debilitating condition with symptoms similar to Long COVID that often occurs after infection. ME/CFS has long been under-recognized and understudied, but there are still lessons from this condition that can inform Long COVID research and lead to answers for both diseases. The review paper points to directions for future study.
- Metformin for Long COVID: One more Long COVID paper: a study published this week in The Lancet shares results from a Long COVID clinical trial at the University of Minnesota, which found that the diabetes drug metformin reduces the risk of developing long-term symptoms when patients take it early in the course of a COVID-19 case. I shared this study when it was first posted as a preprint in March, and also spoke to one of its authors for my STAT/MuckRock story about the RECOVER initiative. I’m glad to see that the major findings haven’t changed in this peer-reviewed version; metformin appears to be a promising treatment option, though more study is needed.
- At-home test receives FDA approval: This week, the FDA approved an at-home, rapid COVID-19 test made by the company Cue Health. It’s the first at-home test to receive full approval, as these tests have previously received Emergency Use Authorization under public health emergency rules. With the federal emergency over, the FDA is encouraging test companies to apply for full approval so that at-home COVID-19 tests can be distributed (and marketed) like other commonly-available health products. The emerency authorizations still apply for tests that don’t have full approval yet, though.
- COVID-19 Medicaid rules led to more coverage for children: For the first three years of the pandemic, federal rules tied to the public health emergency forbid states from kicking their residents off of Medicaid. The policy led to a significant increase in Americans with health insurance—and that includes children, according to a new paper published this week in Health Affairs. For states that changed their Medicaid rules for children due to the pandemic policy, coverage increased by about 5% from 2019 to 2021, representing thousands of kids who were able to get healthcare more easily. Of course, these kids and their family members are now likely to lose their health insurance, as the federal policy ended in April.
- Animal behaviors changed during 2020 lockdowns: Remember when, in the early days of the pandemic, big cities with more stringent lockdowns saw more wild animals than normal? A new paper from a large coalition of scientists, published this week in Science, finds that this pattern wasn’t just anecdotal: animal behavior really did change. The scientists compiled a large dataset of animals tracked with GPS, representing 2,300 individuals from 43 different mammal species, and compared their behaviors in spring 2020 to the same period in 2019. Animals living in areas under strict lockdowns were more likely to travel outside their normal ranges, the researchers found.
-
COVID source shout-out: Body Politic
Body Politic, a health justice organization that has led Long COVID organizing over the last three years, shut down its Slack support group this week. The group has been a valuable place for long-haulers to connect and find resources; it’s also helped launch other important projects, such as the Patient-Led Research Collaborative and the Long COVID Survival Guide.
The organization isn’t ending its support of long-haulers, though: it’s partnered with New Health, a mobile app developed to continue community Long COVID support. “New Health will be hiring Body Politic moderators and board members as their first paid staff, and members of our community are currently testing their app,” Body Politic leaders wrote in an April blog post describing the transition.
I’ve written previously about Body Politic’s fundraising efforts as the group sought to transition form a grassroots, all-volunteer organization to a format that was more sustainable, and I’m glad to see that the group’s leaders have found this solution. But it’s a bit sad to see the original Slack group close—the end of an era.
Thank you to all the volunteers who made the Body Politic group possible, from a journalist who has relied on many of its members and resources in my reporting on Long COVID!
-
Sources and updates, May 28
- New Long COVID papers from the Patient-Led Research Collaborative: Speaking of new Long COVID research: the Patient-Led Research Collaborative, a group of long-haulers who do and support research on their condition, has recently published two new papers. The first, published in Nature and based on a patient survey, discusses Long COVID’s intersection with common psychiatric conditions such as depression and anxiety. The second, published in Fronteirs in Rehabilitation Science, is a review paper going over the reproductive health impacts of Long COVID. Long COVID frequently causes disruptions to the menstrual cycle, gonad function, fertility, and other areas of reproductive health, yet these symptoms are understudied.
- FDA fully approves Paxlovid: The FDA has provided full approval to Pfizer for its antiviral COVID-19 pill, Paxlovid. Millions of Americans have received Paxlovid since it earned Emergency Use Authorization in late 2021, and many studies have shown that it’s effective in reducing the risk of severe COVID-19 symptoms. With the federal public health emergency’s end, the FDA has encouraged pharmaceutical companies to apply for full approval for their COVID-19 products so that they can permanently remain on the market; Paxlovid is a high-profile example of that trend.
- Bivalent COVID-19 vaccines protect, but wane: The CDC published another study this week evaluating the bivalent (or Omicron-specific) COVID-19 booster shots. These vaccines clearly provide additional protection against severe COVID-19 symptoms, the study finds, but this immune system boost goes away after several months. In the study, vaccine effectiveness against hospitalization declined from 62% in early weeks post-vaccination, to 24% at three to six months post-vaccination. The study shows that additional boosters and/or newer vaccines are needed for vulnerable adults.
- Value of regular testing for controlling outbreaks: Another notable new study: researchers at the University of Wyoming compared how well different mitigation strategies work for preventing the spread of COVID-19 and other diseases, using a model informed by both epidemiological and economic factors. They found that frequent testing—paired with isolation for people who tested positive—was more effective at reducing disease spread than physical distancing measures, like closing businesses or having employees work from home. The paper suggests that testing can help reduce illness while keeping businesses open.
- Funding for a WHO disease surveillance initiative: The Rockefeller Foundation and World Health Organization recently announced a new partnership, with the foundation providing $5 million to support the WHO’s Hub for Pandemic and Epidemic Intelligence. This Hub was established in 2021, with goals including fostering global collaboration on disease surveillance, providing better (and more complete) data, and improving tools for public health decisions. Rockefeller’s support will help with scaling up genomic surveillance, real-time data collection, and more.
-
What the new RECOVER study does—and doesn’t—tell us about Long COVID
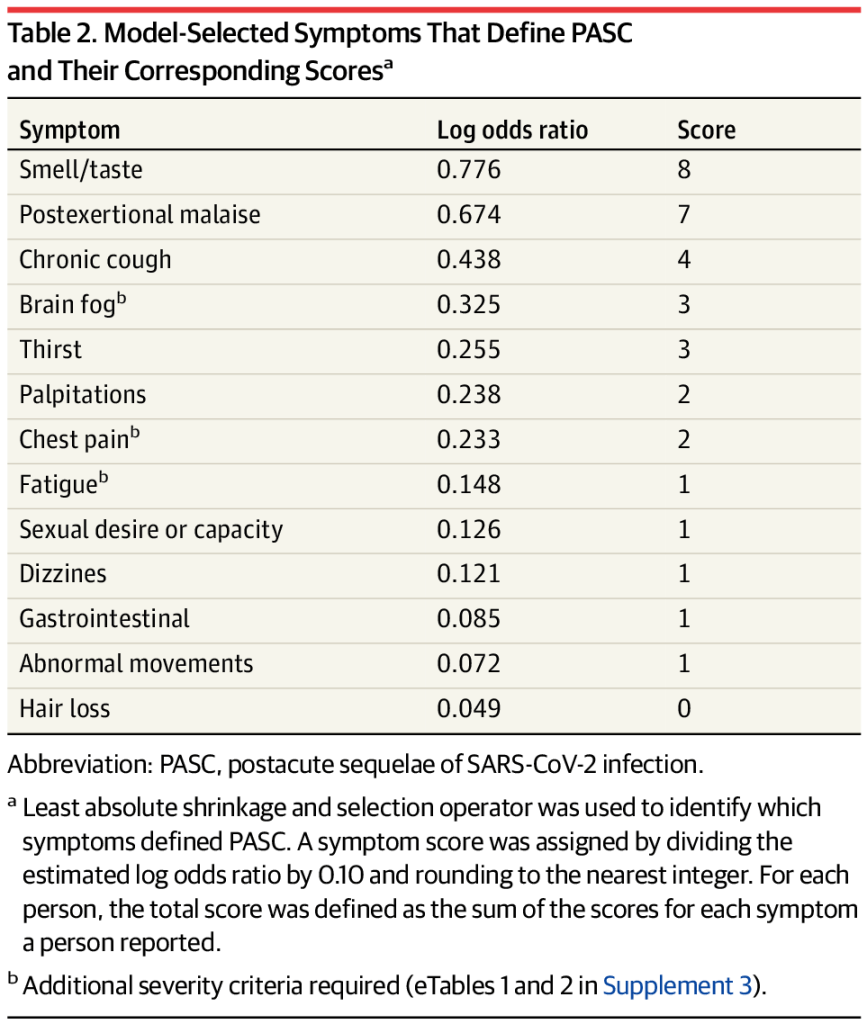
The new RECOVER paper identifies 12 major Long COVID symptoms, but this is far from an exhaustive list defining the condition. RECOVER, the U.S.’s largest initiative to understand Long COVID, published a major scientific study this week in JAMA. The paper goes over key Long COVID symptoms and other findings from nearly 10,000 adults who have joined the project’s research cohort. Its authors propose a new, more specific definition for Long COVID, which will be used in future studies from this project.
This is a big milestone for RECOVER; it’s the first paper to actually share data from the study’s patient cohort, rather than from electronic health records. While the paper doesn’t provide any truly novel, previously unreported information about Long COVID, it confirms findings from smaller studies and validates patient experiences. It’ll certainly be valuable for thousands of scientists around the country struggling to understand this debilitating condition, though patients have expressed some concerns about the paper’s central framework.
This study is also a rather late milestone, considering that the National Institutes of Health received $1 billion in funding from Congress for this initiative in December 2020 (two and a half years ago), and started enrolling patients in fall 2021. For more details on RECOVER’s delays—and many criticisms it’s faced from patients and experts—please check out this investigation by me and Rachel Cohrs, at STAT News and MuckRock.
Here are a few key things you should know about the paper, and that you should watch out for while reading other articles about it:
- The authors’ main goal was to determine which symptoms can specifically be used to diagnose long-term symptoms following a coronavirus infection. Using survey data from RECOVER’s participants, the researchers developed a framework that sorts patients into three categories: definitely having symptoms due to a past coronavirus infection (or “infected”), not having symptoms due to a past infection (“uninfected”), and possibly having symptoms due to a past infection (“unspecified”). This framework prioritizes symptoms unique to Long COVID, such as the loss of smell or taste, over those symptoms that are actually more debilitating for patients, such as chest pain or chronic fatigue. Also, as patient-expert Lisa McCorkell pointed out on Twitter, the unspecified category includes people with Long COVID.
- The paper highlights 12 Long COVID-specific symptoms; there are a lot more. I’ve seen some articles covering this study frame it as, “these are the 12 definitive symptoms of Long COVID.” That’s a misrepresentation of the results. In fact, the authors selected 12 common symptoms that are helpful for their framework (i.e. determining who had a coronavirus infection); we know from other research that Long COVID can include up to 200 different symptoms. RECOVER leader Leora Horwitz even acknowledged in a Twitter thread describing the paper that “these are not the ONLY symptoms that people have, nor are they necessarily the most important to patients, the most common, the most severe or most burdensome.”
- Long COVID is a spectrum, with different clusters of symptoms. This paper adds more evidence to support a hypothesis I’ve heard from many experts, that Long COVID is not one condition but a variety of overlapping conditions all caused by SARS-CoV-2. Different symptoms might be caused by different biological processes, and different patient groups could require different treatments. RECOVER has identified potential patient groups, which the researchers will study further (including through clinical trials, projected to start this summer).
- Long COVID has a wide variety of impacts on day-to-day life, but the most severe patients might not have the best proof. Using this paper’s framework, long-haulers can give themselves a “Long COVID score” reflecting how likely they are to have symptoms caused by a past coronavirus infection. But, as patient-expert Chris Maddison explained, a higher score doesn’t necessarily mean Long COVID has more drastically impacted the patient’s day-to-day life. “I would prefer to flip this, i.e., a def. that centers folks who are suffering regardless of whether we can accurately predict prior infection,” he wrote.
- Infection post-vaccination or with Omicron can lead to lower—but still significant—Long COVID risk, compared to earlier in the pandemic. Since RECOVER started recruiting in fall 2021, the study includes some people who were first infected during the first Omicron wave, then developed Long COVID symptoms afterward. About 10% of the patients infected during Omicron later developed symptoms, which pretty close to the study’s overall estimate of Long COVID prevalence (also about 10%). Vaccination or infection with an Omicron variant may make you less likely to get Long COVID, this study suggests, but the risk is still very present.
- Repeat infections may increase Long COVID risk. RECOVER was able to follow 2,150 people who got infected during the Omicron wave, including 81 who had multiple infections. Of those with multiple infections, 16 people—or one in five—had Long COVID symptoms within six months. That’s double the prevalence rate of those who just had one infection in the same timeframe (10%). While these are small numbers, the finding is certainly worth further study; see this thread from patient-expert Hannah Davis for more details.
- This is not a prevalence paper, and it does not provide a clinical definition of Long COVID. Some media coverage might suggest that this paper has “defined Long COVID,” which is a misrepresentation of the study. While the authors do propose a new framework for evaluating potential Long COVID patients, they make it clear that a lot more research and iteration will be needed before any RECOVER findings should be used in the doctor’s office. The paper also doesn’t provide a definitive answer on how many people get Long COVID, since it includes a relatively small number of people who were uninfected when they joined the study. Quoting Lisa McCorkell again: “It is very clear throughout the paper that in order for this to be actionable at all, iterative refinement is needed.”
- This won’t be the last paper sharing findings from the RECOVER cohort. This study presented data from patients’ symptom surveys, which is just one small part of the RECOVER cohort’s activities. The enrolled patients have also undergone extensive medical testing and symptom tracking over time, which will be the subject of future studies—and will be used to refine RECOVER’s Long COVID framework. Clinical trials will (eventually) provide more data as well.
To my fellow journalists covering this study: I highly encourage you to present this paper as a small part of a complicated, iterative research process, rather than a definitive answer to long-standing questions about Long COVID. I also encourage you to talk to patient-experts and ask for their criticisms of the study (like those I’ve cited here), rather than just letting the RECOVER leadership go unchallenged.
More Long COVID reporting
-
Sources and updates, May 14
- CDC updates ventilation guidance: On Friday, the CDC made its first-ever official air quality recommendation for all indoor spaces, in an update to its overall ventilation guidance. The agency now says all buildings should strive for five air changes per hour (ACH) at a minimum; in other words, clean air should circulate through the space every 12 minutes or more. This update is a victory for many clean air advocates who’ve pushed for better guidelines during the pandemic as a way to reduce the risk of COVID-19 and other respiratory pathogens. As expert and advocate Devabhaktuni Srikrishna said to me on Twitter: “This is exactly the clarity we were pushing CDC for for since last year… Now the question becomes, how does everyone do it in their home, school, and office? How much does it cost? Where do you get it?”
- Millions Missing in Washington, D.C.: On Friday, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and Long COVID patient advocates held a demonstration at the National Mall in Washington, D.C. to show U.S. leaders how chronic disease has pulled millions of Americans out of public life. The demonstration, organized by ME Action and Body Politic, included an installation of 300 cots with hand-made pillowcases created by patients across the country. Each cot is intended to represent people who can no longer work or do other day-to-day activities that were routine before they got sick with Long COVID or a similar chronic illness. You can learn more by watching ME Action’s press conference from the demonstration.
- Post-PHE prices for COVID-19 testing: Researchers at the Kaiser Family Foundation put together a new report describing how much Americans will likely pay for PCR and at-home tests now that the federal government no longer supports blanket insurance coverage. At-home test prices range from $6 to $25 per test, depending on the brand and number of tests purchased at once, the KFF analysis found based on a variety of data sources. PCR tests and others performed in healthcare settings range from $25 to $150 per test, with medians around $50. Tests including COVID-19 and other pathogens are the priciest.
- Sleep apnea and Long COVID risk: A new paper, published this week in the journal SLEEP, finds that people with sleep apnea have a higher risk of developing Long COVID compared to those who don’t have this condition. Researchers at New York University (and other institutions) compared Long COVID symptoms among adults and children with and without sleep apnea through multiple electronic health record databases, finding people with sleep apnea had up to a 75% higher risk of long-term COVID-19 symptoms. This study was supported by the National Institutes of Health’s RECOVER initiative. Like other papers to come out of RECOVER (including another recent study looking at comorbidities), it’s utilized health records rather than the actual cohort of patients recruited into the NIH’s research program.
- Diagnosing COVID-19 through breath: Another notable recent paper, published in the Journal of Breath Research in April: researchers at the University of Colorado Boulder and the National Institute of Standards and Technology have found they can identify whether a patient has COVID-19 by testing their breath. The technique involves using sensitive lasers and artificial intelligence to differentiate between chemicals in a patient’s breath; it’s similar to a breathalyzer for alcohol testing, though more complicated. In addition to COVID-19, breath testing might help identify other diseases.
-
Sources and updates, May 7
- KFF Medicaid Unwinding tracker: The Kaiser Family Foundation just published a new tracker detailing Medicaid enrollment by state. Enrollment rose to record levels during the pandemic, as a federal measure tied to the public health emergency forbid states from taking people off the insurance program. Now, states are going through the slow process of evaluating people’s eligibility and taking some off the program, in a process called “unwinding.” The KFF tracker is following this process, presenting both Medicaid enrollment data by state and information on each state’s timeline for evaluation.
- Biden administration ends vaccine mandates: In time with the federal public health emergency’s end, the Biden administration has announced that it will lift its COVID-19 vaccine rules for federal workers and contractors. International travelers to the U.S. also will no longer need to provide proof of their vaccination status, and the administration is working to end requirements for other groups of workers and travelers. This change is, essentially, another signal of the administration giving up on mass vaccination campaigns; after all, most of the people who got their shots under these rules haven’t received an Omicron booster.
- Vaccine protection wanes over time: A new review paper from researchers in Trento, Italy, published this week in JAMA, shows the importance of booster shots for maintaining protection from COVID-19. The researchers compiled and analyzed findings from 40 studies that evaluated vaccine effectiveness. Overall, they found, the protection that both primary series and booster shots provide against an Omicron infection drops significantly by six months and nine months after vaccination. Remember: Americans over 65 and/or immunocompromised, you’re now eligible for another bivalent/Omicron-specific booster.
- Disparities in COVID-19 deaths persist: Two new studies this week examine COVID-19 deaths by race and ethnicity. The first study, from the CDC’s National Center for Health Statistics, examined deaths of all causes during the pandemic, finding that Black and Native Ameircans had higher death rates than other racial/ethnic groups. COVID-19 was the fourth highest cause of death in 2022, after heart disease, cancer, and unintentional injury. The second study, from Andrew Stokes and collaborators, examined COVID-19 deaths during the U.S.’s first Omicron wave compared to earlier surges, finding that disparities decreased—but only because white deaths went up during the second year of the pandemic.
- Characterizing Long COVID neurological symptoms: Another new study from this week: researchers at the NIH performed detailed examinations of 12 Long COVID patients to better understand their neurological symptoms. The researchers used an approach called “deep phenotyping,” which involves a variety of tests that aren’t typically used in clinical settings. They found that the patients had a number of abnormalities in their immune systems and autonomic nervous systems compared to healthy controls, pointing to different potential drivers of symptoms.
- FDA approves RSV vaccine: Finally, a bit of non-COVID good news: for the first time, the FDA has approved a vaccine for RSV, the seasonal respiratory virus that can cause severe symptoms in older adults and young children. This vaccine, made by GSK, was approved for adults ages 60 and up and will likely get distributed during the next cold/flu season. Scientists have been working on RSV vaccines for decades, making this a major milestone for reducing the disease’s impact. Helen Branswell at STAT has more details.
-

The NIH has little to show for $1 billion allocated to Long COVID research

Article header from STAT News; illustration by Mike Reddy. In December 2020, Congress gave the National Institutes of Health $1.2 billion to study Long COVID. That money was used to fund the RECOVER initiative, billed as a thorough study of this condition and an effort to help patients actually recover from the often-debilitating long-term effects of COVID-19.
But it’s been more than two years, and the RECOVER initiative doesn’t have much to show for that money—besides a growing number of frustrated people in the Long COVID community. Clinical trials haven’t started yet, very limited research findings have been published, and some long-haulers involved with the initiative are losing faith in its ability to find answers.
I collaborated with Rachel Cohrs, a reporter at STAT News, on a thorough investigation into RECOVER’s problems. We combed through documents and data, talked to a number of people involved with the initiative, and researched the broader context around RECOVER.
This project was a collaboration between STAT and MuckRock, and you can read the full story on STAT’s website or on MuckRock’s. I also wrote a Twitter thread with some highlights:
As I wrote on Twitter, I want to keep reporting on RECOVER, as I know there are other problems with the initiative that weren’t captured in this story. If anyone reading this has additional information to share, please shoot me an email or reach out on social media. (You can also reach out to ask for my number on Signal, a secure messaging platform.)
Here’s the story’s introduction, to give you an idea of what we found:
The federal government has burned through more than $1 billion to study long Covid, an effort to help the millions of Americans who experience brain fog, fatigue, and other symptoms after recovering from a coronavirus infection.
There’s basically nothing to show for it.
The National Institutes of Health hasn’t signed up a single patient to test any potential treatments — despite a clear mandate from Congress to study them. And the few trials it is planning have already drawn a firestorm of criticism, especially one intervention that experts and advocates say may actually make some patients’ long Covid symptoms worse.
Instead, the NIH spent the majority of its money on broader, observational research that won’t directly bring relief to patients. But it still hasn’t published any findings from the patients who joined that study, almost two years after it started.
There’s no sense of urgency to do more or to speed things up, either. The agency isn’t asking Congress for any more funding for long Covid research, and STAT and MuckRock obtained documents showing the NIH refuses to use its own money to change course.
“So far, I don’t think we’ve gotten anything for a billion dollars,” said Ezekiel Emanuel, a physician, vice provost for global initiatives, and co-director of the Healthcare Transformation Institute at the University of Pennsylvania. “That is just unacceptable, and it’s a serious dysfunction.”
Eric Topol, the founder and director of the Scripps Research Translational Institute, said he expected the NIH would have launched many large-scale trials by now, and that testing treatments should have been an urgent priority when Congress first gave the agency money in late 2020.
“I don’t know that they’ve contributed anything except more confusion,” Topol said.
Patients and researchers have already raised alarms about the glacial pace of the NIH’s early long Covid efforts. But a new investigation from STAT and the nonprofit news organization MuckRock, based on interviews with nearly two dozen government officials, experts, patients, and advocates, and internal NIH correspondence, letters, and public documents, underscores that the NIH hasn’t picked up the pace — instead, the delays have compounded.
It’s difficult to pinpoint exactly why progress is so stalled, experts and patients involved in the project emphasized, because the NIH has obscured both who is in charge of the long Covid efforts and how it spent the money. The broader Biden administration has also missed opportunities for oversight and accountability of the effort — despite the president’s lofty promises to focus on the disease.
The NIH’s blunders have massive ramifications for the more than 16 million Americans suffering from long Covid, in addition to those with other, similar chronic diseases. As the biggest government-funded study on this topic, the NIH initiative, dubbed RECOVER, sets precedents for future research and clinical guidelines. It will dictate how doctors across the country treat their patients — and, in turn, impact people’s ability to access work accommodations, disability benefits, and more.
“The NIH RECOVER study is pointless,” said Jenn Cole, a long Covid patient based in Brooklyn, N.Y., who wanted to enroll in the study but found the process inaccessible. The research is “a waste of time and resources,” she said, and fails to use patients’ tax dollars for their benefit.
In response to STAT and MuckRock’s questions, the NIH and an institute at Duke University managing the clinical trials defended the initiative, without providing a clear explanation for the delays.
The NIH said it chose to fund a large-scale research program instead of small-scale studies to make sure data and processes could be shared across different groups of patients, adding that clinical trials will be launching soon. In these trials, standardized study designs will allow the agency to test multiple treatments across multiple sites. If there are signals a drug works, the agency said it can pivot to devote more resources there.
A Department of Health and Human Services spokesperson said the agency has made progress over the last year in responding to long Covid, and that there are research efforts underway in addition to the RECOVER program.
“The Administration remains committed to addressing the longer-term impacts of the worst public health crisis in a century,” HHS said.
Read the full story on STAT’s website or on MuckRock’s.
More Long COVID reporting
-
Sources and updates, April 16
- Long COVID care access challenges: A new paper, published this week in JAMA Network Open, shares the results of a survey by the Urban Institute think tank. The researchers surveyed about 9,500 adults, including 800 with self-reported Long COVID, about their experiences accessing medical care. The long-haulers were more likely to report difficulties with accessing and paying for care, compared to adults who don’t have the condition. To address this issue, the healthcare system needs to develop clinical guidelines for Long COVID, train workers about it, address insurance barriers, and more, the researchers said.
- PolyBio announces Long COVID research agenda: Speaking of Long COVID: the PolyBio Research Foundation, a nonprofit devoted to Long COVID, ME/CFS, and other chronic conditions, has announced several research projects that it’s supporting. The projects will evaluate potential biological mechanisms underlying Long COVID symptoms, such as virus persisting in different parts of the body, changes in T cell activity, microclots, and more. PolyBio has a great reputation for pushing ahead post-viral disease research, and I’m looking forward to seeing the results of these studies.
- Bivalent boosters hold up against XBB variants: Another new study that caught my attention this week: researchers at the University of North Carolina and North Carolina state health department reported on how well the bivalent, Omicron-specific boosters worked, based on the agency’s surveillance data. The study examined data from September 2022 through February 2023, a period when the BQ and XBB subvariants were dominating coronavirus spread. North Carolina residents who received the bivalent boosters were significantly less likely to experience severe COVID-19 symptoms, the researchers found, but their protection started to wane within a month after receiving the shots.
- Resources on indoor air quality in schools: Journalist’s Resource recently updated this list of research and resources for journalists interested in covering indoor air quality in K-12 schools. The update follows a CDC report showing that many public schools across the U.S. have failed to upgrade their ventilation, despite federal funding to do so (which I covered last week). School air quality is a topic that deserves more reporting, especially from local journalists who can dig into how their school districts are doing.
- Arizona county starts monitoring for a fungus in wastewater: I’m always on the lookout for new uses of wastewater surveillance, and one promising application could be tracking Candida auris, a fungal pathogen that’s resistant to common drugs and spreads quickly in healthcare settings. The Arizona state health department and a lab at the University of Arizona recently launched a pilot program to track this fungus through Yuma County’s wastewater. Arizona and neighboring southwest states have been a hotbed for C. auris; if this pilot is successful, other states could start similar efforts.