- COVID-19 Vaccine Rollout across US Congressional Districts: This dashboard, from the Harvard Center for Population and Development Studies, breaks down the U.S. vaccine rollout by a geography I haven’t yet seen: Congressional districts. The interactive maps highlight the partisan nature of this vaccine rollout—as POLITICO reporters pointed out in an article citing the tracker, “All but one of the 39 congressional districts where at least 60 percent of residents have received a coronavirus shot are represented by Democrats.”
- Vaccination time series from the CDC: The CDC’s vaccine dashboard doesn’t allow users to download time series data (i.e. vaccinations by day), but now, you can find this information on the agency’s data portal. The dataset includes county-level vaccine administrations and coverage rates going back to December 13, 2020.
- Methodology changes for Bloomberg’s COVID-19 Vaccine Tracker: Bloomberg’s vaccine tracker is one of the most widely cited in the U.S., and for good reason—it’s incredibly comprehensive, compiling data from every country with an active vaccine rollout (and, in the early weeks of the rollout, from every U.S. state). After months of collecting data by hand, the Bloomberg team is now starting to automate their data collection, Health Editor Drew Armstrong announced this week. Many countries and the WHO are now providing stable enough data sources that such a change is possible.
- Fiscal accountability for COVID-19 responses: The International Budget Partnership, a global nonprofit working to improve government budgets, has produced a report and interactive website analyzing accountability measures that international governments have—and have not—implemented as part of emergency COVID-19 responses. Notably, out of 120 countries surveyed, none have “substantive” accountability and only four have “adequate” accountability. (H/t Data Is Plural.)
- COVID-19 risk levels for kid-related activities: This one isn’t a data source, per se, but I thought readers might find it helpful. A team of epidemiologists, immunologists, and public health scientists—including Katelyn Jetelina of Your Local Epidemiologist—compiled this detailed guide for families with unvaccinated children. The guide aims to help parents and families navigate their risk levels this summer.
Category: Vaccines
-
Featured sources, June 13
-
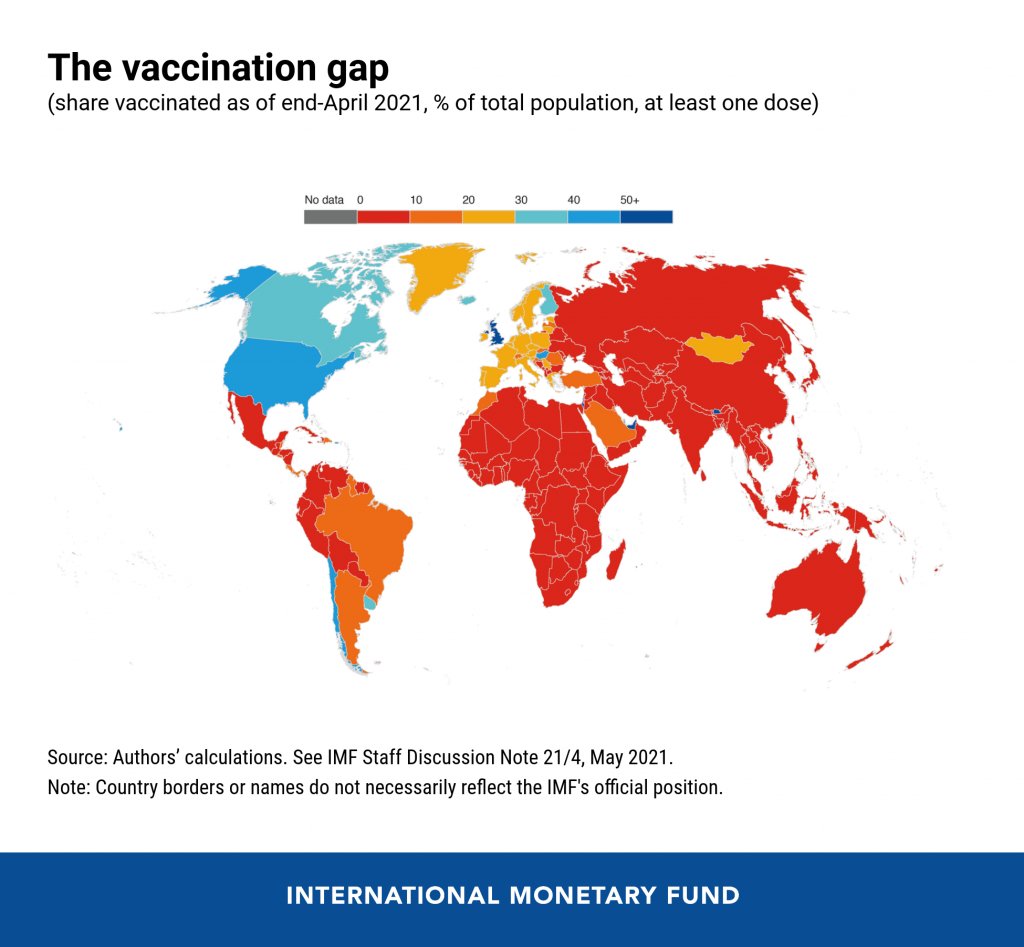
25 million doses is a drop in the global vaccination bucket

The vaccination gap, based on IMF data. Posted on Twitter by Gavin Yamey. In the reader survey I sent out a few weeks ago, I asked, “What is one question you have about COVID-19 in the U.S. right now?” One reader responded with an inquiry into vaccine equity: “What will it look like when the U.S. is ‘open’ and vaccinated and many other parts of the world are not?”
That question feels especially relevant this week. On Thursday, the Biden administration made a big (and long-awaited) announcement: the federal government is sending 25 million vaccine doses from America’s stockpile to other countries. The administration has previously promised to send at least 80 million doses abroad by the end of June, but this week’s announcement included more details—such as countries that will receive these initial doses and other logistics.
Out of the 25 million, about 19 million doses are going to COVAX. COVAX, a global effort run by the World Health Organization and other international government bodies and philanthropic organizations, brings vaccines to low-income nations at no cost. The COVAX doses will go to India, other parts of Asia, Central and South America, and Africa, Bloomberg’s Josh Wingrove reports.
The remaining 6 million doses will be sent directly to countries, including Ukraine, Kosovo, Haiti, Georgia, Egypt, Jordan and Iraq. Some doses are going directly to India as well—while the worst of this nation’s surge may be over, it’s still facing high case counts, full hospitals, and a terrifying “black fungus” linked with the Delta variant (B.1.617).
At first glance, this might seem like a noble move on the Biden administration’s part. The U.S. is seeing low case numbers and widespread reopenings, so we can share some supplies to “help the pandemic around the globe,” as COVID-19 response coordinator Jeff Zients said at a briefing on Thursday.
But 25 million doses—or even the 80 million doses that the administration has promised by the end of this month—is a drop in the bucket compared to actual international needs. For example: COVAX needs 1.8 billion doses to vaccinate about half the adult population in low-income countries. COVAX has specifically prioritized 92 low-income nations, representing a total population of 3.8 billion.
That 1.8 billion dose number is a highlight of a major report released last week by the Rockefeller Foundation, a global charitable foundation, discussing what it would take to vaccinate the world. I covered the report for Science News. According to this report, Gavi (the Vaccine Alliance), an international public-private body that runs COVAX, needs to raise $9.3 billion in order to pay for those 1.8 billion doses. Gavi has been working to raise this money from countries and independent donors at a global health summit this past week.
While $9.3 billion might seem like a massive price tag, the cost of failing to provide these vaccines would actually be far greater. The global economy may lose up to $9.2 trillion if richer nations fail to support equitable vaccine distribution, according to an estimate from the International Chamber of Commerce.
So far, the U.S. has administered about 300 million vaccine doses (as of yesterday), covering over half the total population. In a number of low-income countries, less than one percent of the population has received a dose. Tedros Adhanom Ghebreyesus, Director General of the WHO, said at a recent assembly that, if all doses administered globally had been sent out equitably, the doses would have covered “all health workers and older people.” Instead, high-income nations are largely protected while low-income nations are vulnerable to future surges and highly-transmissible variants.
Through this lens, the 25 million dose shipment announced this week is far from impressive. It’s a useful start, certainly, but it’s not going to end the pandemic anywhere. Even the 80 million doses promised by the end of June is a tiny number—about 4% of the doses COVAX is hoping to obtain. It’s also only 11% of the doses that vaccine makers have pledged to deliver to the U.S. by the end of July, according to Bloomberg.
That larger June shipment has also been held up because the Biden administration is planning to send AstraZeneca vaccines—which are under review from the FDA because they were produced at the Emergent factory that infamously wasted millions of Johnson & Johnson doses. The AstraZeneca vaccine is not authorized for use in the U.S., so of course it will make up the majority of the doses we send abroad this summer.
Speaking of unused doses: the Biden administration may also start sending unused doses from states to other countries, POLITICO reported this week. The administration wants to get thousands of Pfizer, Moderna, and J&J doses—on the verge of expiring—to countries that would actually use them. While this possible policy hasn’t yet been realized, it hammers home a clear message: the U.S. will donate “unwanted” vaccine doses only when we are absolutely certain that we don’t need them here.
Now, let’s return to our reader’s question. What happens when the U.S. is safely vaccinated, but other parts of the world aren’t?
From a health standpoint, the U.S. will probably be okay. The vaccines are very effective, even against variants—likely protecting the country from another major surge. We will need careful surveillance to guard against future variants that may evolve beyond the vaccines (see: last week’s issue), and it’s possible that overly zealous reopening this summer will lead to outbreaks next fall and winter. But seniors and other vulnerable people would be more protected than they have been in past surges, and booster shots (for the variants) will likely be on their way soon. In short, America’s wealth will protect us.
Around the world, however, outbreaks will continue. Every time a new person gets infected with the coronavirus, the virus has a new opportunity to mutate. And with every mutation, the virus learns to spread faster, to evade common treatments, even to evade vaccines. Thanks to globalization, as long as the virus is a threat anywhere, it continues to be a threat everywhere.
Plus, as low-income nations suffer from continued outbreaks, the global economy will continue to suffer. Out of that $9.2 trillion cost estimated by the International Chamber of Commerce, the majority will likely fall on wealthier nations (like the U.S.) that rely on other countries for products and labor.
“The pandemic itself has gone beyond a health crisis — it has now gone into an economic crisis,” Christy Feig, the Rockefeller Foundation’s director of communications and advocacy, told me when I spoke to her for Science News. “The only way to unchoke the economy is by getting the vaccines to as many countries as possible, so that we can stop the spread of the disease before more variants come.”
(P.S. If you’d like to read more on how the pandemic may end in the U.S. and elsewhere, I recommend this story by STAT’s Helen Branswell.)
More international reporting
-
Moderna for the middle children
Good news for kids hoping for jabs in arms (which used to sound like an oxymoron before this spring): Moderna has announced promising results for its trial in adolescent-aged children. In around 4,000 adolescents, the vaccine proved to be 94.1% effective in preventing disease. No cases in the vaccinated group were found two weeks after the second shot, while 4 cases were found in the unvaccinated control group.
On Tuesday, May 25, Moderna showed in a clinical trial that its mRNA vaccine is safe and effective in people ages 12 to 17. The company will apply for FDA emergency use authorization in June. This follows the semi-recent authorization of the Pfizer-Biontech vaccine for the same age group, which happened at the end of March.
While children tend to have less severe complications from COVID-19 on the whole, serious illness is still quite possible. And even though rates across the country are falling due to more widespread vaccination, it’s still important that kids get vaccinated as herd immunity is not quite in our grasp yet.
The availability of another vaccine may help more people in this age group get protected; however, the rest of the world has nowhere near the access to vaccines that U.S. citizens over age 12 do right now. In April, health policy experts estimated that the United States might have an excess of up to 300,000 extra vaccines.
That being said, adolescents should still get vaccinated if it is available to them. This problem isn’t the fault of citizens wanting to get protection; it’s about the failures of governments and systems to provide vaccine equity.
More vaccine reporting
- Sources and updates, November 12Sources and updates for the week of November 12 include new vaccination data, a rapid test receiving FDA approval, treatment guidelines, and more.
- How is the CDC tracking the latest round of COVID-19 vaccines?Following the end of the federal public health emergency in May, the CDC has lost its authority to collect vaccination data from all state and local health agencies that keep immunization records. As a result, the CDC is no longer providing comprehensive vaccination numbers on its COVID-19 dashboards. But we still have some information about this year’s vaccination campaign, thanks to continued CDC efforts as well as reporting by other health agencies and research organizations.
- Sources and updates, October 8Sources and updates for the week of October 8 include new papers about booster shot uptake, at-home tests, and Long COVID symptoms.
- COVID source shout-out: Novavax’s booster is now availableThis week, the FDA authorized Novavax’s updated COVID-19 vaccine. Here’s why some people are excited to get Novavax’s vaccine this fall, as opposed to Pfizer’s or Moderna’s.
- COVID-19 vaccine issues: Stories from COVID-19 Data Dispatch readers across the U.S.Last week, I asked you, COVID-19 Data Dispatch readers, to send me your stories of challenges you experienced when trying to get this fall’s COVID-19 vaccines. I received 35 responses from readers across the country, demonstrating issues with insurance coverage, pharmacy logistics, and more.
- Sources and updates, November 12
-

Why did the CDC change its breakthrough case reporting?
Earlier this month, the CDC made a pretty significant change in how it tracks breakthrough cases. Instead of reporting all cases, the agency is only investigating and collecting data on those cases that result in hospitalizations or deaths.
In case you need a refresher: “breakthrough cases” are those infections that occur after a patient is fully vaccinated (including both doses, if applicable, and the two-week waiting period after a final dose). These cases are rare—like, one in ten thousand rare. As I wrote back in April, it’s important to contextualize any reporting on these cases with their incredible rareness so that we hammer home just how effective the vaccines are.
But just because breakthrough cases are rare doesn’t mean we shouldn’t pay attention to them. In fact, it’s critical to pay attention to these cases in order to monitor precisely how well our vaccines are working—and how new variants may threaten the protections those vaccines provide.
As The Atlantic’s Katherine J. Wu explains:
Breakthroughs can offer a unique wellspring of data. Ferreting them out will help researchers confirm the effectiveness of COVID-19 vaccines, detect coronavirus variants that could evade our immune defenses, and estimate when we might need our next round of shots—if we do at all.
As I’ve discussed in past variant reporting, numerous studies have demonstrated that the vaccines currently in use in the U.S.—especially the Pfizer and Moderna vaccines—work well against all variants. That includes variants of concern, such as B.1.617 (from India), B.1.351 (from South Africa), and P.1 (from Brazil). But the vaccine efficacy rates for some of these variants are lower than that stellar 95% we saw in Pfizer and Moderna’s clinical trials. And some common therapeutic drugs don’t work well for patients infected with variants, too.
As a result, scientists are concerned that, while the vaccines are working well now, they might not work well forever. Whenever the coronavirus infects a new person, it has the opportunity to evolve. And that continued evolution must be monitored. The first coronavirus variant able to evade our vaccines may emerge in a foreign country with a raging outbreak—but it may also emerge here in the U.S. Closely monitoring all breakthrough cases will help us find that dangerous variant.
(Of note: A new, potentially-concerning variant was identified just last night in Vietnam; WHO scientist Maria Van Kerkhove described it as an offshoot of the variant from India, B.1.617, with “additional mutation(s).”)
With that in mind, let’s unpack the CDC’s reporting change. When the vaccine rollout started, the agency was investigating all breakthrough cases that came to its attention—including those in patients with only mild symptoms, or with no symptoms at all. According to an agency study released this past Tuesday, the CDC identified 10,262 such breakthrough cases from 46 U.S. states and territories between January 1 and April 30, 2021.
Keep in mind: By April 30, about 108 million Americans had been fully vaccinated. Dividing 10,262 by 108 million is where I got that “one in ten thousand” comparison I cited earlier. As I said: very rare.
Starting on May 1, however, the CDC changed its strategy. Now, it is only tracking breakthrough cases that result in severe illness for patients, leading to hospitalization and/or death. The CDC says that this choice is intended to focus on “the cases of highest clinical and public health significance” rather than tracking down asymptomatic cases.
In its May 25 report, CDC scientists said that 27% of the breakthrough cases identified before May 1 were asymptomatic. 10% of the infected individuals were hospitalized, though almost a third of those patients were hospitalized for a reason unrelated to COVID-19. Only 160 patients (less than 2% of the breakthrough cases) died.
We need to take these numbers with a grain of salt, though, because the CDC has likely undercounted the true number of asymptomatic cases. Both clinical trials and studies on vaccine effectiveness in the real world have suggested that those people who get infected with COVID-19 after completing a vaccination regime are more likely to have mild symptoms, or no symptoms at all.
Plus, the CDC is recommending that vaccinated Americans don’t need to get tested before traveling, if they have come into contact with someone known to have COVID-19, or for many of the other reasons that many of us got tested this past year. (The agency is still recommending that fully vaccinated people get tested if they’re experiencing COVID-19 symptoms, though.)
As I wrote at Slate Future Tense last month, such guidelines are likely to drive down the number of COVID-19 tests conducted across the U.S. And this trend seems to be happening, so far: PCR tests dropped from their winter surge levels this spring, and are now dropping again. (Antigen and other rapid tests may be getting used more, but we don’t have any comprehensive data on them.)
With that drop in testing—combined with the overall challenge of identifying asymptomatic COVID-19 cases outside of dedicated studies—it would be pretty damn hard for the CDC to track down all breakthrough cases. The agency’s focus on more serious cases instead may thus be considered a conservation of resources, directing research efforts and care to those Americans who get seriously ill after vaccination.
But “a conservation of resources” is also a nice way of saying, the CDC made a lazy choice here. The agency has poured money into genomic surveillance over the past few months, sequencing over 20,000 cases a week (compared to a few thousand cases a week before Biden took office). In recent weeks, the Biden administration has announced renewed funding for public health and similar commitments to prioritizing scientific research. If the CDC wants to find and sequence breakthrough cases in order to identify vaccine-busting variants, there should be nothing stopping the agency.
Or, as epidemiologist Dr. Ali Mokdad told the New York Times: “The C.D.C. is a surveillance agency. How can you do surveillance and pick one number and not look at the whole?”
Out of those 10,262 cases that the CDC reported this week, only 5% had sequence data available—but the majority of those sequined cases were variants of concern, including B.1.1.7 and P.1. At The Atlantic, Wu reported that epidemiologists in some parts of the country are seeing more breakthrough cases tied to concerning variants, while others are seeing breakthrough case sequences that match the overall infections in the community.
To me, this high level of unknowns and uncertainties mean that we need more breakthrough case reporting and sequencing, not less. And we need a national public health agency that commits to true surveillance, so that we aren’t flying blind when the coronavirus inevitably evolves beyond our current defenses.
(P.S. Shout-out to Illinois, the one state that reports its own breakthrough case data.)
More vaccine reporting
- Sources and updates, November 12Sources and updates for the week of November 12 include new vaccination data, a rapid test receiving FDA approval, treatment guidelines, and more.
- How is the CDC tracking the latest round of COVID-19 vaccines?Following the end of the federal public health emergency in May, the CDC has lost its authority to collect vaccination data from all state and local health agencies that keep immunization records. As a result, the CDC is no longer providing comprehensive vaccination numbers on its COVID-19 dashboards. But we still have some information about this year’s vaccination campaign, thanks to continued CDC efforts as well as reporting by other health agencies and research organizations.
- Sources and updates, October 8Sources and updates for the week of October 8 include new papers about booster shot uptake, at-home tests, and Long COVID symptoms.
- COVID source shout-out: Novavax’s booster is now availableThis week, the FDA authorized Novavax’s updated COVID-19 vaccine. Here’s why some people are excited to get Novavax’s vaccine this fall, as opposed to Pfizer’s or Moderna’s.
- COVID-19 vaccine issues: Stories from COVID-19 Data Dispatch readers across the U.S.Last week, I asked you, COVID-19 Data Dispatch readers, to send me your stories of challenges you experienced when trying to get this fall’s COVID-19 vaccines. I received 35 responses from readers across the country, demonstrating issues with insurance coverage, pharmacy logistics, and more.
- Sources and updates, November 12
-
Vaccine cocktails look viable—just in time for hot-vax summer
Some good global vaccine news this week: it looks like vaccine cocktails may be a promising option.
A clinical trial based in Spain of around 600 participants (aged 18-59) reported encouraging results regarding mix-and-match vaccines (or “heterologous prime-and-boost,” if you want the jargon) meaning one shot of one vaccine and the second shot of another. In this study, the first dose given was AstraZeneca, and the second was Pfizer.
The study found that protective IgG antibodies were 30-40 times higher in the treatment group than the control group (those who had only received the first dose of the AstraZeneca vaccine). Neutralizing antibodies were also seven times higher after the Pfizer dose compared to the control, while usually they double in number after the second AstraZeneca shot.
As some people familiar with Covid vaccines may note, these vaccines use two different mechanisms to stimulate the immune system: the AstraZeneca shot uses an adenovirus vector modified with the SARS-CoV-2 spike protein while the Pfizer vaccine uses messenger RNA to coax cells into making the spike protein themselves. This early success demonstrates that vaccines with different mechanisms can be combined to induce a strong immune response.
In the wake of the AstraZeneca blood clot news, it’s reasonable to expect that some may be hesitant to get the second shot if they have received the first AstraZeneca shot. Some authorities have advised people who have gotten the first dose of AstraZeneca to get an alternative for the second shot. Having an alternative that hasn’t been linked to blood clots might persuade those hesitant to get the second AstraZeneca shot to complete a vaccination regimen, especially if it might stimulate even more of an immune response than the regular AstraZeneca regimen.
There’s currently another heterologous prime-and-boost trial in place in the United Kingdom with a slightly more complicated experimental setup (the four groups were AstraZeneca for both shots, Pfizer for both shots, Pfizer for the first and AstraZeneca for the second, or vice versa), with all participants over 50.
This study hasn’t reported results regarding immune responses yet, but they have reported some preliminary reactogenicity results. On May 12, researchers reported that mild side effects like fever or fatigue were more common in people who had received mixed vaccines. However, there were no severe side effects, and the mild ones subsided after a few days. The Spanish study did not find this, and instead found that mild side effects were about as common as they were with a regular vaccine regimen.
The UK study is expected to report immune response data soon, so it’ll be interesting to see if it matches the results found by the Spanish study. We’ll keep you updated when those results come out.
More vaccine reporting
- Sources and updates, November 12Sources and updates for the week of November 12 include new vaccination data, a rapid test receiving FDA approval, treatment guidelines, and more.
- How is the CDC tracking the latest round of COVID-19 vaccines?Following the end of the federal public health emergency in May, the CDC has lost its authority to collect vaccination data from all state and local health agencies that keep immunization records. As a result, the CDC is no longer providing comprehensive vaccination numbers on its COVID-19 dashboards. But we still have some information about this year’s vaccination campaign, thanks to continued CDC efforts as well as reporting by other health agencies and research organizations.
- Sources and updates, October 8Sources and updates for the week of October 8 include new papers about booster shot uptake, at-home tests, and Long COVID symptoms.
- COVID source shout-out: Novavax’s booster is now availableThis week, the FDA authorized Novavax’s updated COVID-19 vaccine. Here’s why some people are excited to get Novavax’s vaccine this fall, as opposed to Pfizer’s or Moderna’s.
- COVID-19 vaccine issues: Stories from COVID-19 Data Dispatch readers across the U.S.Last week, I asked you, COVID-19 Data Dispatch readers, to send me your stories of challenges you experienced when trying to get this fall’s COVID-19 vaccines. I received 35 responses from readers across the country, demonstrating issues with insurance coverage, pharmacy logistics, and more.
- Sources and updates, November 12
-
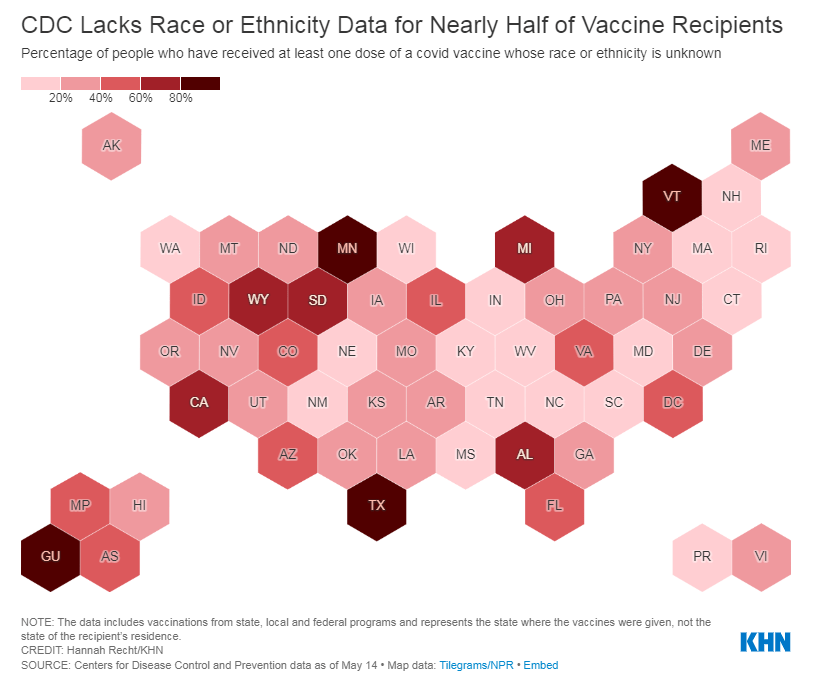
The CDC needs to release state-by-state data on who’s getting vaccinated
For months, I’ve been calling on the CDC to release state-by-state demographic data on who is getting vaccinated. While the vast majority of states report this information themselves, the state data are completely unstandardized—making it difficult to perform comprehensive analyses or compare one state to another.
“The vaccine data that individual states are publishing replicate the patchwork nature of the other state-level COVID-19 data our teams have been compiling,” COVID Tracking Project leaders Alice Goldfarb and Erin Kissane wrote in The Atlantic in January.
While many more states are reporting vaccination demographics now than in January—Montana and Wyoming are the only two states that now fail to report vaccinations by race—the data continue to be patchwork and hard to analyze.
Bloomberg has devoted a small team to analyzing and presenting these data in the publication’s U.S. Vaccine Demographics Tracker. But Bloomberg isn’t making their underlying data public, so other journalists and researchers are unable to build on this work. And really, it shouldn’t be on journalists to standardize from a fragmented state-by-state landscape—it should be the work of the CDC.
That’s why I was thrilled when, this week, we finally got that data from the CDC. Well… sort-of.
A team from KHN received CDC state-by-state demographic vaccination data via a public records request. This team—which includes Hannah Recht, Rachana Pradhan, and Lauren Weber—analyzed the CDC’s data and made their work public on GitHub.
The data indicate that, despite promises from the White House to prioritize vulnerable communities in the vaccination campaign, a lot of inequities persist: “KHN’s analysis shows that only 22% of Black Americans have gotten a shot, and Black rates still trail those of whites in almost every state.”
!function(){“use strict”;window.addEventListener(“message”,(function(a){if(void 0!==a.data[“datawrapper-height”])for(var e in a.data[“datawrapper-height”]){var t=document.getElementById(“datawrapper-chart-“+e)||document.querySelector(“iframe[src*=’”+e+”‘]”);t&&(t.style.height=a.data[“datawrapper-height”][e]+”px”)}}))}();In some states, white residents have been vaccinated at almost twice the rate of Black residents. In Iowa, for example, 15% of the Black population has received at least one dose—compared with 37% of the white population. Other states with high disparities include Florida, New Hampshire, Maine, Wisconsin, New Jersey, New York, Hawaii, and Connecticut.
Hispanic/Latino vaccination rates also lag behind the rates for white residents in some states, but the disparities are not as drastic as those for the Black population. Nationwide, 22% of Black Americans have received at least one dose, compared to 33% of white Americans.
Both Native Americans and Asian Americans have higher vaccination rates than the white population. Many tribes, in particular, have made dedicated efforts to promote vaccination.
And another hopeful caveat: vaccination rates for minorities have improved in recent weeks as the rate for white Americans goes down. In the last two weeks, about half of first doses administered in the U.S. have gone to people of color. This includes about 24% of doses going to Hispanic/Latino Americans, 10% going to Black Americans, and 8% going to Asian Americans.
The day after KHN’s analysis was published, Victoria Knight (another KHN reporter) asked CDC Director Dr. Rochelle Walensky whether the agency would add state-level race and ethnicity vaccination data to its dashboard.
“We have been updating our website,” Dr. Walensky said in response. “I can’t say that it’s daily; I believe that it’s weekly.”
And yet as of Sunday morning, May 23, state-by-state demographic data are nowhere to be found on the CDC’s site.
Knight also asked what the CDC is doing to address the high number of vaccinations for which demographic details are unknown. Race/ethnicity data are missing for about 44% of vaccinated Americans, meaning that true disparities may be even starker.
!function(){“use strict”;window.addEventListener(“message”,(function(a){if(void 0!==a.data[“datawrapper-height”])for(var e in a.data[“datawrapper-height”]){var t=document.getElementById(“datawrapper-chart-“+e)||document.querySelector(“iframe[src*=’”+e+”‘]”);t&&(t.style.height=a.data[“datawrapper-height”][e]+”px”)}}))}();In some states, that unknown percentage is much higher than 44%. Eight states “either refuse to provide race and ethnicity details to the CDC or are missing that information for more than 60% of people vaccinated,” according to KHN. These states are excluded from KHN’s analysis as a result: they are Alabama, California, Michigan, Minnesota, South Dakota, Texas, Vermont and Wyoming.
Dr. Walensky told reporters the CDC is working with state and local public health departments to improve demographic reporting, but didn’t provide specifics.
In order to continue improving vaccination rates for minority communities, the CDC needs to actually make all of the agency’s data public. If state-by-state demographic data were easily available, researchers and reporters like me could more easily identify both the success stories and the disappointments—and help the states that are lagging catch up.
As Hannah Recht put it on Twitter: “we should not have to keep FOIAing for CDC state-level data that they could just put online if they wanted to.”
More vaccine reporting
- Sources and updates, November 12Sources and updates for the week of November 12 include new vaccination data, a rapid test receiving FDA approval, treatment guidelines, and more.
- How is the CDC tracking the latest round of COVID-19 vaccines?Following the end of the federal public health emergency in May, the CDC has lost its authority to collect vaccination data from all state and local health agencies that keep immunization records. As a result, the CDC is no longer providing comprehensive vaccination numbers on its COVID-19 dashboards. But we still have some information about this year’s vaccination campaign, thanks to continued CDC efforts as well as reporting by other health agencies and research organizations.
- Sources and updates, October 8Sources and updates for the week of October 8 include new papers about booster shot uptake, at-home tests, and Long COVID symptoms.
- COVID source shout-out: Novavax’s booster is now availableThis week, the FDA authorized Novavax’s updated COVID-19 vaccine. Here’s why some people are excited to get Novavax’s vaccine this fall, as opposed to Pfizer’s or Moderna’s.
- COVID-19 vaccine issues: Stories from COVID-19 Data Dispatch readers across the U.S.Last week, I asked you, COVID-19 Data Dispatch readers, to send me your stories of challenges you experienced when trying to get this fall’s COVID-19 vaccines. I received 35 responses from readers across the country, demonstrating issues with insurance coverage, pharmacy logistics, and more.
- Sources and updates, November 12
-
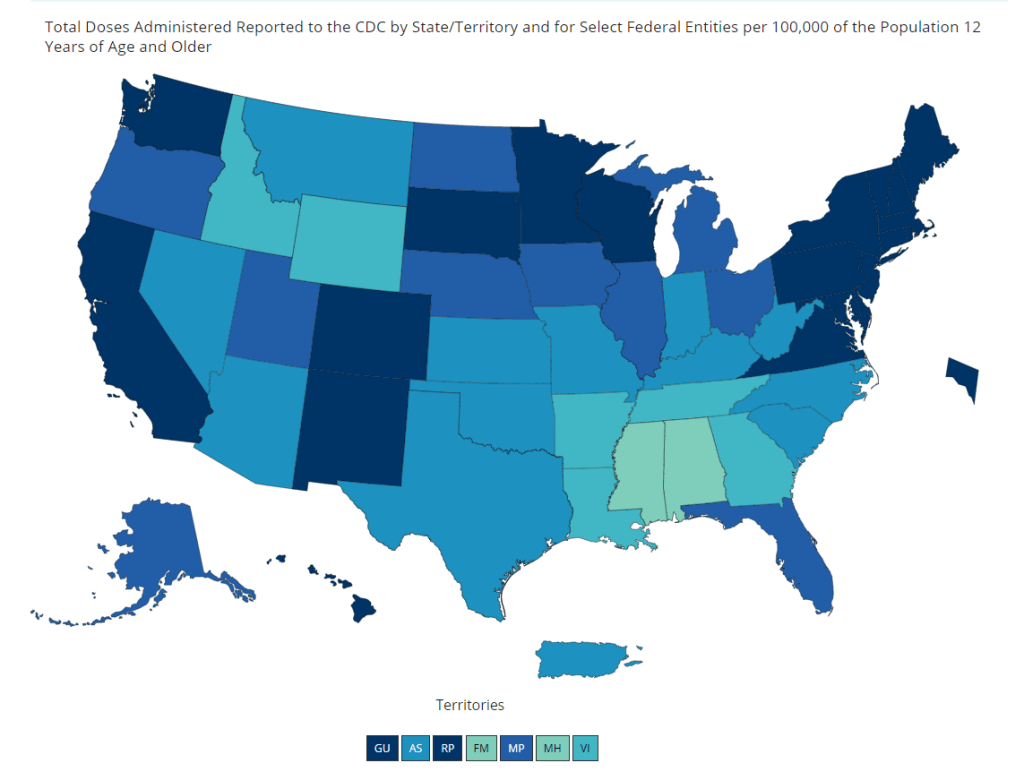
Pfizer for the whole pfamily (now CDC-verified!)
In case you missed it amidst the mask discourse: Pfizer was already the “vaccine for cool people,” but this week, it formally became the vaccine for teens. The FDA announced on Monday that it was expanding the Emergency Use Authorization for this vaccine to include children ages 12 to 15, and the CDC followed this up with an official recommendation on Wednesday.
As Sarah Braner reported when the Pfizer adolescent trial results were released: “In the trial, no participants who received the vaccine contracted symptomatic COVID-19 out of a total of 2,260 participants, marking an efficacy rate of 100%.” So, this formal endorsement was a pretty foregone conclusion, but it’s still good news for the 17 million children ages 12 to 15 in the country.
Here are a couple more statistics about the 12-15 age group, via the Kaiser Family Foundation:
- This group accounts for 5% of the U.S. population and 27% of the population under age 16.
- Nearly half of children in this age group are people of color, including: 25% are Hispanic, 13% are Black, and 5% are Asian.
- 36% of children in this age group live in a family with incomes below 200% of the Federal Poverty Level.

Vaccination coverage for the population ages 12 and up, as of May 15. The darker blue a state is, the better its coverage. And speaking of adolescent data: on Friday, the CDC diversified its vaccine tracker. In addition to state-by-state views of vaccination coverage for the overall population, adult population, and senior population, the Tracker will now show you vaccination coverage for each state’s population over age 12. Nationwide, 56% of this group has had at least one dose and 44% is fully vaccinated.
The Vaccinations County View page will show you coverage over age 12 by county, but these data aren’t yet available for easy download in the Community Profile Reports.
The CDC’s demographic vaccination data, meanwhile, groups adolescents in with (already eligible) 16 to 18-year olds in an under 18 category—so we aren’t yet able to see precisely how many children in this age group are getting vaccinated. This may become a concerning data gap as schools may seek to use 12-15 vaccination rates as an indicator for reopening next fall.
More vaccine coverage
- Sources and updates, November 12Sources and updates for the week of November 12 include new vaccination data, a rapid test receiving FDA approval, treatment guidelines, and more.
- How is the CDC tracking the latest round of COVID-19 vaccines?Following the end of the federal public health emergency in May, the CDC has lost its authority to collect vaccination data from all state and local health agencies that keep immunization records. As a result, the CDC is no longer providing comprehensive vaccination numbers on its COVID-19 dashboards. But we still have some information about this year’s vaccination campaign, thanks to continued CDC efforts as well as reporting by other health agencies and research organizations.
- Sources and updates, October 8Sources and updates for the week of October 8 include new papers about booster shot uptake, at-home tests, and Long COVID symptoms.
- COVID source shout-out: Novavax’s booster is now availableThis week, the FDA authorized Novavax’s updated COVID-19 vaccine. Here’s why some people are excited to get Novavax’s vaccine this fall, as opposed to Pfizer’s or Moderna’s.
-

The data behind the CDC’s new mask guidance
This past Thursday, the CDC announced that, if you are fully vaccinated, the pandemic is basically over for you.
The announcement surprised everyone—from my parents to COVID-19 scientists—because it appeared to come out of nowhere. Before this week, the CDC’s most recent guidance revisions included an acknowledgment that surface transmission of the coronavirus is very rare and a recommendation that masks might not be necessary outside; both of these conclusions were already well-known in the scientific community. In fact, STAT published an article last week in which Nicholas Florko suggests that the CDC’s COVID-19 messaging has been “overly cautious” and perhaps even “irrelevant for most Americans.”
So, what led to the announcement on Thursday? The rest of this post will go over the CDC’s evidence for its guidance, taking the epidemiological perspective. Also, as two-thirds of Americans aren’t yet fully vaccinated, I’ll touch on another COVID-19 truism that has garnered some confusion lately: yes, you are significantly safer outside than you are inside.

CDC graphic on COVID-19 safety, updated with the new mask guidance on Thursday. (I need to acknowledge, though, that a. there are certainly outside political and economic forces at play here, and b. the public health perspective on this guidance is far more complicated. For one perspective on the public health side, I recommend this Twitter thread by virologist Angela Rasmussen.)
Our first category of evidence: the mRNA vaccines work really well. It’s no surprise that the Pfizer and Moderna vaccines are both exceptionally capable of protecting people against the coronavirus, but a couple of recent studies really hammer this home:
- In this recent study from Israel, the Pfizer vaccine demonstrated 97% effectiveness against symptomatic cases and 86% effectiveness against asymptomatic cases among about 6,700 healthcare workers who were regularly tested for COVID-19.
- According to this MMWR report from the CDC, the Pfizer and Moderna vaccines were 94% effective in preventing COVID-19 hospitalization for fully vaccinated seniors (over age 65), demonstrating how well the vaccines protect against severe disease. Plus, the vaccines were 64% effective in preventing hospitalization for partially vaccinated seniors.
- Another MMWR report, released this past Friday, demonstrates that the mRNA vaccines were 94% effective at preventing symptomatic COVID-19 in U.S. healthcare workers. A single shot of Pfizer or Moderna ws 82% effective at preventing symptomatic COVID-19.
- A study from the Cleveland Clinic, a medical research center based in Cleveland, Ohio, studied COVID-19 cases among its caregivers after vaccines were made available; the Clinic found that a whopping 99.7% of those workers who tested positive for COVID-19 had not been fully vaccinated. Only 0.3% were breakthrough cases. Meanwhile, 99.75% of COVID-19 patients that the Clinic treated during the study’s time frame were not fully vaccinated.
- According to the CDC’s breakthrough case data, out of about 122 million fully vaccinated Americans, less than 1,400 people have been hospitalized or have died due to COVID-19. That’s 0.001%. (The CDC’s breakthrough reporting focuses on severe outcomes rather than cases, as these cases may be difficult to systematically identify outside of studies.)
Second evidence category: the vaccines work against variants.
- It’s important to note here that, when I say “vaccines work,” it’s not an all or nothing situation. A vaccine might protect you against severe COVID-19 disease or death (the primary goal), but not against an asymptomatic case that allows you to transmit the virus to someone else.
- All the COVID-19 vaccines currently on the market protect us against severe COVID-19 disease and death—whether you’re infected with an older version of the coronavirus or with a variant.
- For a couple of the variants, that protection might not be quite as secure. Studies on B.1.351 (the variant first identified in South Africa), P.1 (identified in Brazil), and B.1.617 (identified in India) have all demonstrated lower vaccine effectiveness. But again, lower effectiveness here still means protection for the majority of people who get vaccinated.
- And one big advantage of mRNA vaccine technology is, the Pfizer and Moderna vaccines may easily be adjusted to protect against particularly concerning variants. Moderna recently reported promising data for two booster shots designed to protect specifically against B.1.351 and P.1.
- If you’d like a more detailed rundown of the vaccine v. variants battle, check out this article by STAT’s Andrew Joseph.
Third evidence category: U.S. cases are way down.
- As I noted in today’s National Numbers post: cases have dropped by 50% in the last month. And beyond that, cases have dropped from a peak of 250,000 new cases per day in January to under 40,000 new cases per day now.
- During this time frame, most states did not impose lockdowns or other restrictions on the level of what we saw in spring 2020. So, these drops can primarily be attributed to the vaccines.
- We still do not have much evidence showing how well the vaccines protect against asymptomatic transmission and infection, but the evidence we have so far is promising, as Apoorva Mandavilli explains in the New York Times. (One major study investigating this question in college students is currently underway.)
- Still, the massive case drops—occurring even as B.1.1.7 and other more contagious variants spread through the country—indicate that the vaccines must be doing some work to stall coronavirus spread from one person to another. This supports the CDC’s argument that vaccinated Americans can take off their masks in public without worrying about spreading a latent coronavirus to someone else.
Fourth evidence category: outdoor transmission is incredibly low.
- Earlier this week, the New York Times’ David Leonhardt provided a compelling argument for why, though the CDC said “less than 10%” of COVID-19 transmission occurs outside, the true number is actually much lower. In fact, fewer than 1% (and possibly even fewer than 0.1%) of COVID-19 cases happen due to someone getting infected outside.
- As I’ve previously reported, there is not a single recorded superspreading event that took place solely outside. This includes the large Black Lives Matter protests last summer. (A few superspreading events have both outdoor and indoor components.)
- A new study from researchers at Drexel University specifically examined COVID-19 transmission in parks, and found no correlation between the number of people using a park and the number of COVID-19 cases in the surrounding ZIP code. The research suggests that you should feel safe at your local park, even if it seems a bit more crowded and less mask-adherent than it did a few months ago. I spoke to Franco Montalto and Bita Alizadehtazi, two authors on this study, who emphasized that “it’s important to get outside,” take advantage of the green infrastructure in your neighborhood, and feel safe while doing so.
This evidence brings us to what The Atlantic’s Drew Thompson calls the “Two Commandments of COVID-19”:
1. COVID-19 is an indoor aerosol disease.
2. Vaccination protects you; more vaccinations protect everyone.
Speaking just for myself: I am fully vaccinated, but I fully intend to keep wearing a mask in stores, on the subway, and even outside when I’m in a large crowd of people. This is partially because my state still has a mask mandate in place, but also because there are still a lot of people in my community who aren’t yet vaccinated—and I don’t want to pose a risk to them, no matter how small that risk may be. (In Brooklyn, where I live, 41% of the population has had at least one dose and 33% are fully vaccinated, according to city data.)
Suffice it to say, the CDC makes recommendations about COVID-19 safety. It doesn’t issue requirements. I made a personal masking decision for myself, based on the community where I live; I hope this article helped you understand the science behind the guidance change so that you can do the same. And if you have questions—my inbox is always open.
More vaccine coverage
- Sources and updates, November 12Sources and updates for the week of November 12 include new vaccination data, a rapid test receiving FDA approval, treatment guidelines, and more.
- How is the CDC tracking the latest round of COVID-19 vaccines?Following the end of the federal public health emergency in May, the CDC has lost its authority to collect vaccination data from all state and local health agencies that keep immunization records. As a result, the CDC is no longer providing comprehensive vaccination numbers on its COVID-19 dashboards. But we still have some information about this year’s vaccination campaign, thanks to continued CDC efforts as well as reporting by other health agencies and research organizations.
- Sources and updates, October 8Sources and updates for the week of October 8 include new papers about booster shot uptake, at-home tests, and Long COVID symptoms.
- COVID source shout-out: Novavax’s booster is now availableThis week, the FDA authorized Novavax’s updated COVID-19 vaccine. Here’s why some people are excited to get Novavax’s vaccine this fall, as opposed to Pfizer’s or Moderna’s.
- COVID-19 vaccine issues: Stories from COVID-19 Data Dispatch readers across the U.S.Last week, I asked you, COVID-19 Data Dispatch readers, to send me your stories of challenges you experienced when trying to get this fall’s COVID-19 vaccines. I received 35 responses from readers across the country, demonstrating issues with insurance coverage, pharmacy logistics, and more.
-
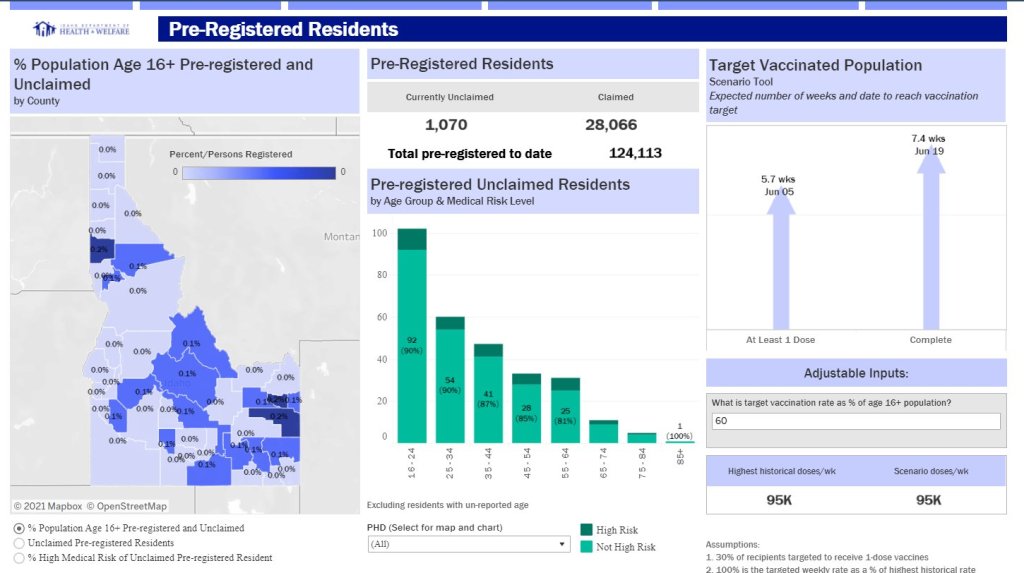
COVID source shoutout: Unique vaccine metrics
Most states report some variation on the same COVID-19 vaccination metrics: doses allocated, doses administered, state residents who’ve been partially and fully vaccinated, and so on. But some states go beyond those basics.
This week, I’m showing some appreciation for:
- Washington D.C.: Reports numbers of District residents who have stuck to their second dose appointments. The dashboard’s “Adherence” tab includes those who are fully vaccinated, waiting on their second dose appointment, or more than a week overdue for that second dose appointment—both District-wide and by ward.
- Idaho: Reports detailed data on state residents who have pre-registered for appointments. On the “Pre-registered residents” tab, you can see how many people have claimed their pre-registered appointments, as well as breakdowns of the pre-registered residents by county, age, and medical risk level.
- Illinois: Reports both a count of unusable vaccine doses and information on vaccine breakthrough cases. The “unusable” vaccine doses count includes doses that have been discarded, dropped, or had some other issue in the storage and handling process. For vaccine breakthroughs, Illinois reports total patients hospitalized and died due to COVID-19 after vaccination.
- New Hampshire: Reports counts of vaccine doses distributed and administered by individual vaccination sites, such as hospitals and public health networks. (New Hampshire includes vaccination data once a week in its COVID-19 news reports, usually on Thursdays. The state figures differ significantly from CDC-reported numbers, for as-yet-undetermined reasons.)
As always, you can find the CDD’s full set of annotations on national and state vaccine data sources here.
-
J&J is back on the menu
After 10 days, the pause on the J&J vaccine has been lifted. According to CDC Director Rochelle Walensky, there have been about 1.9 cases of severe blood clotting per million people who had received the J&J vaccine. It has been re-authorized for use in people aged 18 and older, now with an addendum to the label and fact sheet warning of the risk of blood clots:
It’s important to note that at time of writing (April 24) only some states have already resumed its use. (These are Arizona, Colorado, Connecticut, Louisiana, Maine, Massachusetts, Michigan, Missouri, Nevada, New York, Tennessee, Texas, Indiana, and Virginia.) However, this is coinciding with a larger trend of states ordering fewer vaccine doses.
The J&J vaccine’s return is probably good news for the rest of the world as well. Combined with the AstraZeneca vaccine, the J&J vaccine was supposed to be one of the big players in the global fight against COVID-19. But the U.S. pause raised concerns for vaccine diplomacy and the global rollout—J&J had also paused its European distribution, South Africa announced they were putting J&J distribution on a temporary hiatus, and Australia said it wouldn’t purchase any J&J doses. Resuming distribution in the U.S., which can act as a bellwether for which vaccines are seen as desirable abroad, might allay concerns about safety abroad.
More vaccine coverage
- Sources and updates, November 12Sources and updates for the week of November 12 include new vaccination data, a rapid test receiving FDA approval, treatment guidelines, and more.
- How is the CDC tracking the latest round of COVID-19 vaccines?Following the end of the federal public health emergency in May, the CDC has lost its authority to collect vaccination data from all state and local health agencies that keep immunization records. As a result, the CDC is no longer providing comprehensive vaccination numbers on its COVID-19 dashboards. But we still have some information about this year’s vaccination campaign, thanks to continued CDC efforts as well as reporting by other health agencies and research organizations.
- Sources and updates, October 8Sources and updates for the week of October 8 include new papers about booster shot uptake, at-home tests, and Long COVID symptoms.
- COVID source shout-out: Novavax’s booster is now availableThis week, the FDA authorized Novavax’s updated COVID-19 vaccine. Here’s why some people are excited to get Novavax’s vaccine this fall, as opposed to Pfizer’s or Moderna’s.
- Sources and updates, November 12