
This past Thursday, an advisory committee to the CDC recommended that booster doses of the Pfizer vaccine be authorized for seniors and individuals with high-risk health conditions. The committee’s recommendation, notably, did not include individuals who worked in high-risk settings, such as healthcare workers—whom the FDA had included in its own Emergency Use Authorization, following an FDA advisory committee meeting last week.
Then, very early on Friday morning, CDC Director Rochelle Walensky announced that she was overruling the advisory committee—but agreeing with the FDA. Americans who work in high-risk settings can get booster shots. (At least, they can get booster shots if they previously received two doses of Pfizer’s vaccine.)
This week’s developments have been just the latest in a rather confusing booster shot timeline:
Why has this process been so confusing? Why don’t the experts agree on whether booster shots are necessary, or on who should get these extra shots? Part of the problem, of course, is that the Biden administration announced booster shots were coming in August, before the scientific agencies had a chance to review all the relevant evidence.
But from my (data journalist’s) perspective, the booster shot confusion largely stems from a lack of data on breakthrough cases.
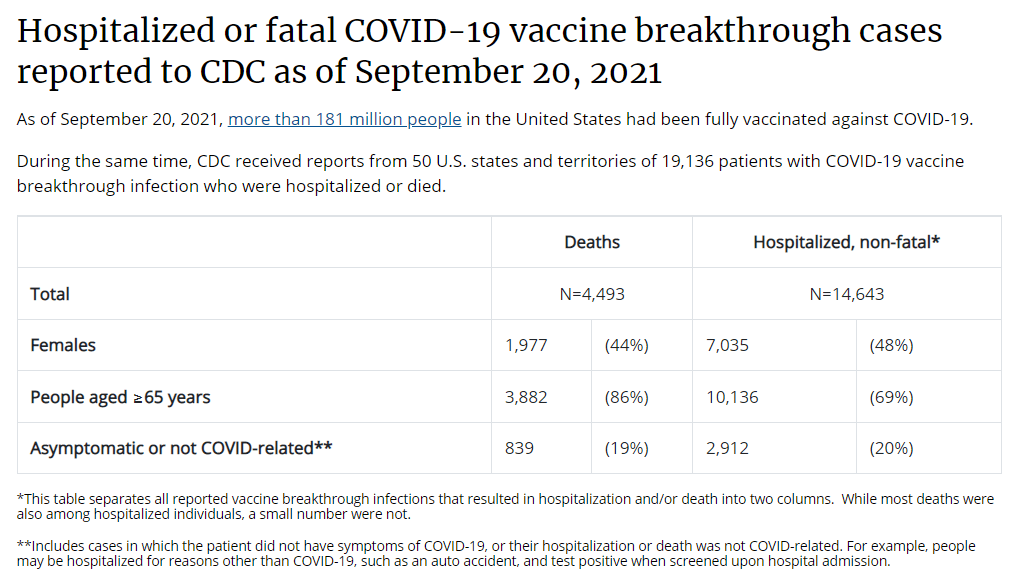
Let’s go back in time—back four months, or about four years in pandemic time. In May, the CDC announced a major change in its tracking of breakthrough cases. The agency had previously investigated and published data on all breakthrough cases, including those that were mild. But starting in May, the CDC was only investigating and publishing data on those severe breakthrough cases, i.e. those which led to hospitalization or death.
At the time, I called this a lazy choice that would hinder the U.S.’s ability to track how well the vaccines are working. I continued to criticize this move, when researchers and journalists attempted to do the CDC’s job—but were unable to provide data as comprehensive as what the CDC might make available.
Think about what might have been possible if the CDC had continued tracking all breakthrough cases, or had even stepped up its investigation of these cases through increased testing and genomic sequencing. Imagine if we had data showing breakthrough cases by age group, by high-risk health condition, or by occupational setting—all broken out by their severity. What if we could compare the risk of someone with diabetes getting a breakthrough case, to the risk of someone who works in an elementary school?
If we had this kind of data, the FDA and CDC advisory committees would have information that they could use to determine the potential benefits of booster shots for specific subsets of the U.S. population. Instead, these committees had to make guesses. Their guesses didn’t come out of nowhere; they had scientific studies to review, data from Pfizer, and information from Israel and the U.K., two countries with better public health data systems than the U.S. But still, these guesses were much less informed than they might have been if the CDC had tracked breakthrough cases and outbreaks in a more comprehensive manner.
From that perspective, I can’t really fault the CDC and the FDA for casting their guesses with a fairly wide net—including the majority of Americans who received Pfizer shots in their authorization. There’s also a logistical component here; the U.S. has a lot of doses that are currently going unused (thanks to vaccine hesitancy), and may be wasted if they aren’t used as boosters.
But it is worth emphasizing how a lack of data on breakthrough cases has driven a booster shot decision based on fear of who might be at risk, rather than on hard evidence about who is actually at risk. Other than seniors; the risk for that group is fairly clear.
The booster shot decision casts a wide net. But at the same time, it creates a narrow band of booster eligibility: only people who got two doses of Pfizer earlier in 2021 are now eligible for a Pfizer booster. Recipients of the Moderna and Johnson & Johnson vaccines are still left in the dark, even though some of those people may need a booster more than many people who are now eligible for additional Pfizer shots. (Compare, say, a 25-year-old teacher who got Pfizer to a 80-year-old, living in a nursing home, with multiple health conditions who got Moderna.)
That Pfizer-only restriction also stems from a data issue. The federal government’s current model for approving vaccines is very specific: first a pharmaceutical company submits its data to the FDA, then the FDA reviews these data, then the FDA makes a decision, then the CDC reviews the data, then the CDC makes a decision.
By starting with the pharmaceutical company, the decision-making process is restricted to options presented by that company. As a result, we aren’t seeing much data on mixing-and-matching different vaccines, which likely wouldn’t be profitable for vaccine manufacturers. (Even though immunological evidence suggests that this could be a useful strategy, especially for Johnson & Johnson recipients.)
In short, the FDA and CDC’s booster shot decision is essentially both ahead of evidence on who may benefit most from a booster, but behind evidence for non-Pfizer vaccine recipients. It’s kind-of a mess.
I also can’t end this post without acknowledging that we need to vaccinate the whole world, not just the U.S. Global vaccination went largely undiscussed at the FDA and CDC meetings, even though it is a top concern for many public health experts outside these agencies.
At an international summit this week, President Biden announced more U.S. donations to the global vaccine effort. His administration seems convinced that the U.S. can manage both boosters at home and donations abroad. But the White House only has so much political capital to spend. And right now, it’s pretty clearly getting spent on boosters, rather than, say, incentivizing the vaccine manufacturers to share their technology with the Global South.
I can only imagine this situation getting messier in the months to come.
Leave a reply to Delta variant and end of social distancing were the biggest reasons why efficacy of COVID-19 vaccines against infection fell by as much as 25% over the summer – not waning of antibodies, New York DOH study finds – newsBlok Cancel reply