In case you missed it amidst the mask discourse: Pfizer was already the “vaccine for cool people,” but this week, it formally became the vaccine for teens. The FDA announced on Monday that it was expanding the Emergency Use Authorization for this vaccine to include children ages 12 to 15, and the CDC followed this up with an official recommendation on Wednesday.
As Sarah Braner reported when the Pfizer adolescent trial results were released: “In the trial, no participants who received the vaccine contracted symptomatic COVID-19 out of a total of 2,260 participants, marking an efficacy rate of 100%.” So, this formal endorsement was a pretty foregone conclusion, but it’s still good news for the 17 million children ages 12 to 15 in the country.
Here are a couple more statistics about the 12-15 age group, via the Kaiser Family Foundation:
- This group accounts for 5% of the U.S. population and 27% of the population under age 16.
- Nearly half of children in this age group are people of color, including: 25% are Hispanic, 13% are Black, and 5% are Asian.
- 36% of children in this age group live in a family with incomes below 200% of the Federal Poverty Level.

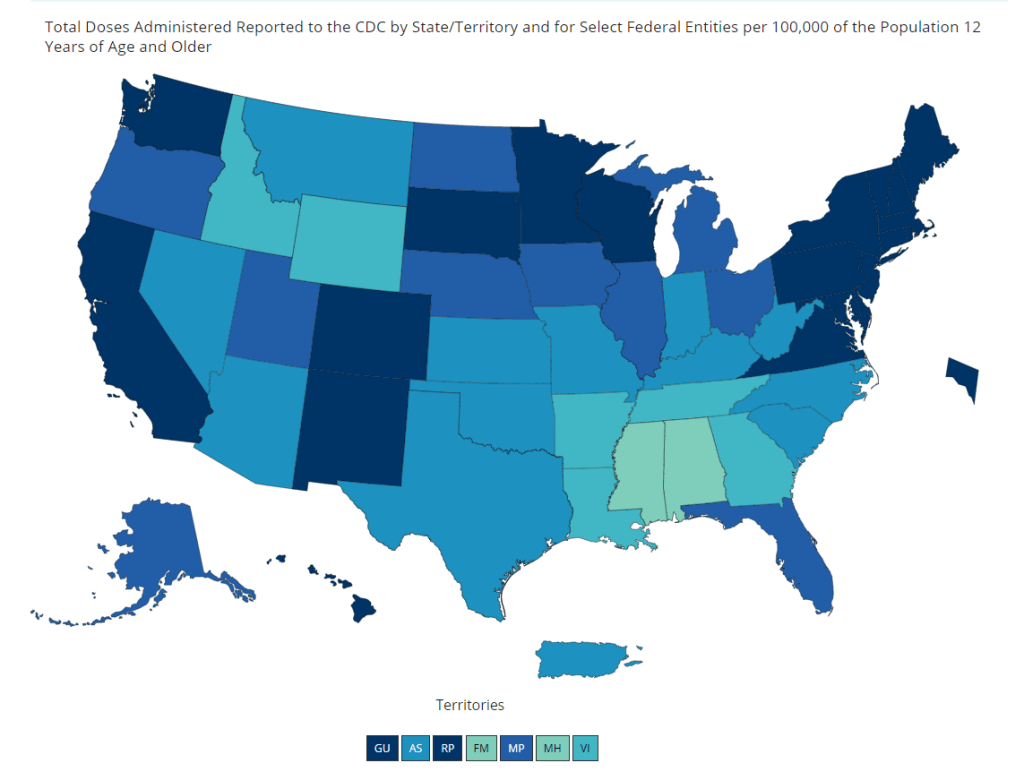
And speaking of adolescent data: on Friday, the CDC diversified its vaccine tracker. In addition to state-by-state views of vaccination coverage for the overall population, adult population, and senior population, the Tracker will now show you vaccination coverage for each state’s population over age 12. Nationwide, 56% of this group has had at least one dose and 44% is fully vaccinated.
The Vaccinations County View page will show you coverage over age 12 by county, but these data aren’t yet available for easy download in the Community Profile Reports.
The CDC’s demographic vaccination data, meanwhile, groups adolescents in with (already eligible) 16 to 18-year olds in an under 18 category—so we aren’t yet able to see precisely how many children in this age group are getting vaccinated. This may become a concerning data gap as schools may seek to use 12-15 vaccination rates as an indicator for reopening next fall.
More vaccine coverage
- Sources and updates, November 12Sources and updates for the week of November 12 include new vaccination data, a rapid test receiving FDA approval, treatment guidelines, and more.
- How is the CDC tracking the latest round of COVID-19 vaccines?Following the end of the federal public health emergency in May, the CDC has lost its authority to collect vaccination data from all state and local health agencies that keep immunization records. As a result, the CDC is no longer providing comprehensive vaccination numbers on its COVID-19 dashboards. But we still have some information about this year’s vaccination campaign, thanks to continued CDC efforts as well as reporting by other health agencies and research organizations.
- Sources and updates, October 8Sources and updates for the week of October 8 include new papers about booster shot uptake, at-home tests, and Long COVID symptoms.
- COVID source shout-out: Novavax’s booster is now availableThis week, the FDA authorized Novavax’s updated COVID-19 vaccine. Here’s why some people are excited to get Novavax’s vaccine this fall, as opposed to Pfizer’s or Moderna’s.
Leave a reply to Moderna for the middle children – Vaccines Cancel reply