- Free at-home tests from the federal government: The Department of Health and Human Services (HHS) and U.S. Postal Service are restarting their program offering free COVID-19 rapid, at-home tests. Starting tomorrow, every U.S. household will be able to order four more tests at covidtests.gov. HHS also announced that it’s buying about 200 million further rapid tests from major manufacturers, paying a total of $600 million to twelve companies. Of course, four tests per household is pretty minimal when you consider all the exposures people are likely to have this fall and winter—but it’s still helpful to see the federal government acknowledge a continued need for testing.
- New grants support Long COVID clinics: The HHS and Agency for Healthcare Research and Quality (AHRQ) also announced a new grant program for clinics focused on Long COVID, aiming to make care for this condition more broadly accessible to underserved communities. Nine clinics across the country have received $1 million each, with the opportunity to renew their grants over the next five years. (At least, that’s my interpretation of the HHS press release, which says $45 million in total is allocated to this program.) This is a pretty significant announcement, as it marks the first time that the federal government is specifically funding Long COVID care; funding has previously gone to RECOVER and other research projects.
- CDC announces new disease modeling network: One more federal announcement: the CDC’s Center for Forecasting and Outbreak Analytics has established a new program to improve the country’s disease surveillance, working with research institutions across the country. The CDC has awarded $262.5 million of funding to the thirteen institutions participating in this program, which it’s calling the Outbreak Analytics and Disease Modeling Network. These institutions will develop new surveillance tools, test them in small-scale projects, and scale up the successful options to broader public health systems. For more context on the CDC’s forecasting center, see my story for FiveThirtyEight last year.
- Testing wildlife for COVID-19: Speaking of surveillance: researchers at universities and public agencies are collaborating on new projects aiming to better understand how COVID-19 is spreading and evolving among wild animals. One project, at Purdue University, is focused on developing a test to better detect SARS-CoV-2 among wild animals. A second project, at Penn State University, is focused on increased monitoring, with plans to test 58 different wildlife species and identify sources of transmission from animals to humans. Both projects received grants from the U.S. Department of Agriculture and involve collaboration with state environmental agencies.
- Paxlovid access falls along socioeconomic lines: A new study, published this week in JAMA Network Open, examines disparities in getting Paxlovid. Researchers at the National Institutes of Health analyzed public data on Paxlovid availability as of May 2023. Counties with higher poverty, less health insurance coverage, and other markers of high socioeconomic vulnerability had significantly less access to Paxlovid than better-off counties, the scientists found. Meanwhile, a separate study (also in JAMA Network Open last week) found that Paxlovid and another antiviral treatment, made by Merck, both remain very effective in reducing severe COVID-19 symptoms. Improving access to these treatments should be a top priority for the public health system.
- Undercounted COVID-19 cases in Africa: One more study that caught my attention this week: researchers at York University in Canada developed a mathematical model to assess how many people actually got COVID-19 in 54 African countries during the first months of the pandemic. Overall, only 5% of cases in these countries were actually reported, the researchers found, with a range of reporting from 30% in Libya to under 1% in São Tomé and Príncipe. A majority of cases in these countries were asymptomatic, the models suggested, indicating many people may not have realized they were infected. The study shows “a clear need for improved reporting and surveillance systems” in African countries, the authors wrote.
Tag: Long COVID
-
Sources and updates, September 24
-
Sources and updates, September 3
- CDC respiratory virus updates: The CDC has a new webpage dedicated to “updates on the respiratory illness season.” So far, it just includes summaries of the agency’s two reports on new variant BA.2.86. Going forward, the page will be updated weekly with further information on COVID-19, flu, RSV, and other viruses spreading this fall and winter.
- Potential biomarker for Long COVID brain fog: A new paper, published this week in Nature by a coalition of researchers in the U.K., connects blood clot issues during acute COVID-19 to cognitive symptoms later on. The researchers found that some patients had low levels of two specific proteins connected to blood clots, based on blood samples taken early in their infections; the same patients were likely to experience brain fog and similar symptoms. If these results are replicated in other studies, the proteins could be used as biomarkers (i.e. medical indicators) of Long COVID symptoms, potentially a big step for research and treatments.
- Long COVID research presented at Keystone Symposia event: Speaking of Long COVID research: scientists gathered to discuss this condition at a conference last week in New Mexico. The conference was hosted by Keystone Symposia, an organization that convenes meetings on important life sciences topics. Highlights from the event included a presentation showing changes to muscle tissue during post-exertional malaise, along with presentations from the Patient-Led Research Collaborative, the National Institutes of Health, Resia Pretorius from Stellenbosch University in South Africa, Akiko Iwasaki from Yale University, and more. I look forward to seeing papers expanding on the talks that occurred at this meeting.
- COVID-19’s impact on Native Americans: Another notable paper from this week examined COVID-19’s disproportionate impacts on Native Americans in New Mexico. Researchers at the University of New Mexico Hospital analyzed patient outcomes in early pandemic waves, from spring 2020 through winter 2021. Compared to white and Hispanic patients, Native Americans were more likely to experience severe COVID-19 outcomes such as more time spent in the hospital and going on a ventilator. “Self-reported AI/AN race/ethnicity emerged as the highest risk factor for severe COVID-19,” the researchers reported, suggesting that this vulnerable group of people deserves additional safety resources.
- COVID-19 burden for cancer patients: One more study to highlight: researchers at Massachusetts General Hospital examined COVID-19 mortality among cancer patients during the first two years of the pandemic, using data from the CDC. People with cancer were more likely to die of COVID-19 during the winter Omicron wave in 2021-2022, compared to the surge during the prior winter (with 18% higher deaths). Meanwhile, deaths among the general population went down from the first to the second winters. Like the study above, this paper suggests that greater protections are needed for vulnerable people during times of high COVID-19 spread. (For example: we could keep masks in healthcare settings!)
-
Sources and updates, August 20
- New toolkit for estimating COVID-19 risk from wastewater: Researchers at Mathematica published a new, open-source toolkit for interpreting wastewater data. It includes an algorithm that scientists and health officials can use to identify when a new surge might be starting based on wastewater results, as well as a risk estimator tool that combines wastewater data with healthcare metrics. The researchers developed this toolkit using data from North Carolina during the Delta and Omicron surges; their paper in PNAS last month describes it further, as does a blog post by the Rockefeller Foundation (which funded the project). This tool doesn’t provide real-time updates, as it only includes wastewater data through December 2022, but it offers a helpful model for using this source to inform public health policies.
- Vaccine delays for uninsured Americans: The CDC estimates that new COVID-19 boosters will become available in late September or early October, as I wrote last week. But Americans without health insurance may have to wait longer to get the shots or pay a hefty price tag, according to recent reporting from POLITICO. A federal government program with national pharmacy chains, which will provide the shots for free to uninsured people, is not slated to start until mid-October. Instead, uninsured people will need to pay out-of-pocket or find one of a small number of federal health centers to get vaccinated; this is likely to discourage vaccinations, POLITICO reports. And the number of uninsured people is only growing thanks to Medicaid redeterminations.
- Budget cuts at the CDC could mean layoffs: A recent op-ed in STAT News, written by two researchers familiar with the CDC’s organizational structure, warns that budget cuts at the agency could lead to a significant reduction in public health workers. The CDC’s budget was cut as part of the federal government’s debt ceiling negotiations last month, the authors explain. It faces a cut of about 10%, or $1.5 million a year, which could lead to significant layoffs. The reduced jobs are particularly likely to impact staff at the state and local levels, the op-ed’s authors argue, rather than at the CDC’d headquarters in Atlanta. “Reductions there will cut public health services and will have their greatest impact on the most vulnerable populations,” they write.
- Vaccine effectiveness for young children: Speaking of the CDC: the agency published a study this week in its Morbidity and Mortality Weekly Report describing COVID-19 vaccine effectiveness for the youngest children who are eligible (i.e. under five years old). Researchers at the CDC and partners at healthcare centers across the country tracked COVID-related emergency department and urgent care visits among young children, from July 2022 through July 2023. Effectiveness for the primary series was low: Moderna’s two-dose series scored just 29% effective at preventing ED and urgent care visits, while Pfizer’s three-dose series was 43% effective. Children who received a bivalent (Omicron-specific) follow-up dose were much more protected, however: this regimen was 80% effective. Bivalent boosers should be a priority for young kids along with adults, the study suggests.
- Immune system changes following COVID-19: Another notable study from this week, from scientists at Weill Cornell Medicine and other institutions, describes how severe COVID-19 cases may damage patients’ immune systems. The researchers analyzed how specific genes were expressed in immune system cells taken from people who had severe cases of COVID-19. They found expression changes as long as a year after patients’ initial infections, and connected those changes to inflammation, organ damage, and other long-term issues. These genetic changes may point to one cause for Long COVID symptoms, though the study is somewhat limited by its focus on patients who had severe symptoms early on (as most people with Long COVID have initially milder cases).
- Smell and taste loss following COVID-19: While smell loss has long been considered a classic COVID-19 symptom, a new study shows that taste loss is also common, even among people who don’t lose their sense of smell. Researchers at the Monell Chemical Senses Center (a nonprofit center in Philadelphia) studied these symptoms through an online survey, which included about 10,000 participants between June 2020 and March 2021. COVID-positive participants were more likely to report smell issues, taste issues, and both together, compared to people who didn’t get sick, the researchers found. Their survey methodology—which included asking people to self-assess their senses by smelling common household objects—could be used for further large-scale studies of these symptoms, the researchers write.
-
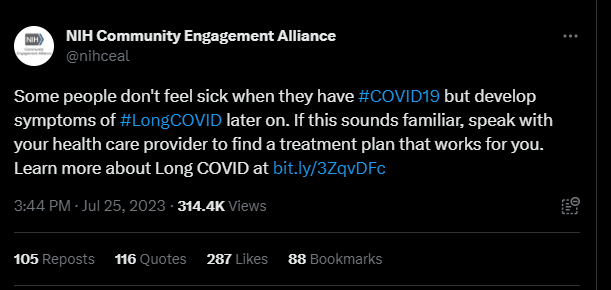
The NIH says it “inappropriately” censored Long COVID patients on social media
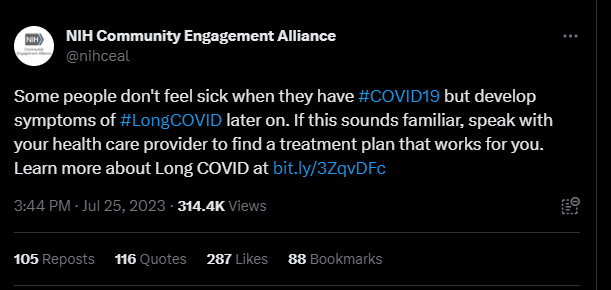
This Tweet, from the NIH’s Community Engagement Alliance, sparked criticism on social media—some of which was hidden by the NIH account. By Miles W. Griffis
The National Institute of Health (NIH) is under fire for censoring comments from patients on social media — the latest in a trend of heavy criticism from people living with Long COVID for failing to listen to patients and implement their input into its $1.15 billion study, RECOVER. Patient concerns have been echoed by both scientists and healthcare professionals who have criticized the study’s lack of results, glacial pace, potentially harmful clinical trials, and wasted funds.
Last month, the NIH Community Engagement Alliance (NIH CEAL) tweeted, “Some people don’t feel sick when they have #COVID19 but develop symptoms of #LongCOVID later on. If this sounds familiar, speak with your health care provider to find a treatment plan that works for you.” The controversial tweet received over one hundred responses, many from people with Long COVID and other infection-associated illnesses.
Patients claimed the post contained misinformation about Long COVID treatments, as this debilitating multi-systemic condition affecting millions does not have any approved treatments or cures. Other commenters shared their negative experiences with their primary health care providers, who they say didn’t offer them any treatment plans or worse, gaslit them and wrote their symptoms off completely. Over 35 of these comments were hidden by the NIH CEAL account.
When asked about this comment hiding, the NIH told me that their social media policy was “overapplied” and that comments on the post were “inappropriately hidden.”
Olenka Sayko, a person with Long COVID whose comment was hidden, said the censorship added to a feeling of hopelessness: “Are we ever going to find solutions for Long COVID if patient voices aren’t being listened to?” She said the censorship is especially concerning since it came from an NIH account dedicated to community engagement. “Who are they engaging with? They’re hiding comments.” Lauren, another person with Long COVID who also was censored, said that the NIH CEAL’s tweet rhetoric sweeps Long COVID and the people experiencing it “under the rug.”
NIH CEAL clarified their tweet earlier this month. “While there’s no cure for Long COVID,” the new post read, “there may be treatment options that can address one’s symptoms & may help people living with Long COVID have better days.”
Although there are no treatments or cures for Long COVID, there are some treatments for conditions associated with or triggered by COVID-19 or Long COVID, including dysautonomia, cardiac disease, diabetes, and others. Many healthcare professionals recommend that patients who have prolonged symptoms following COVID-19 should be screened for life-threatening medical events that can be caused by COVID-19 or Long COVID, including pulmonary embolisms, deep vein thrombosis, or strokes. Long COVID can be fatal. A CDC analysis found that more than 3,500 people have died of the condition, though many experts believe this is a vast undercount.
And while there are many Long COVID clinics around the country that may give the illusion of successful treatment plans, patients often don’t have successful experiences. In an article I wrote for Popular Science, I found that some clinics recommend potentially harmful treatments like graded exercise therapy. Others rejected and gaslit patients. Some only offered generic informational handouts.
During an August 31 NIH RECOVER press conference, the director of the National Institute of Neurological Disorders and Stroke (NINDS), Walter Koroshetz, responded to my question about what Long COVID treatment plans the NIH CEAL account was referring to. He said that the NIH does not make treatment recommendations, adding that the NIH CEAL tweet might have been a misunderstanding. When I asked why the agency was censoring tweets from Long COVID patients, Lawrence Tabek, the acting director of the NIH, said he couldn’t speak to my question and said he has “no idea how social media works”.
I later followed up with the NIH over email about the censored comments. The agency wrote that “The National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities (NIMHD) co-manage the NIH Community Engagement Alliance (CEAL) social media accounts and follow the NHLBI Privacy Statement and Comment Policy.” The policy states commenters should post “on topic,” “be respectful,” and “truthful.” It also prohibits spam and product endorsements.
The NIH wrote over email that, in the case of the censored comments on the July 25th tweet, their policy was “overapplied” and that comments on the post were “inappropriately hidden.” They added that upon further review, comments on the post are now “public,” or unhidden. Some comments, however, were still hidden at the time of publication.
But Eric Goldman, the co-director of the High Tech Law Institute at Santa Clara University School of Law, said this very policy may not be even constitutional. “Assuming that the NIH is a state actor, then anytime they take an action on social media to control the conversation, their decisions are governed by the First Amendment, which protects our right to free speech,” he said.
Instances like the NIH censoring comments on social media are complicated, but upcoming Supreme Court cases may provide some clarity, Goldman said.
Two cases, Lindke v. Freed (from the 6th Circuit Court of Appeals) and Garnier v. O’Connor-Ratcliff (from the 9th Circuit Court of Appeals) may be heard by the Supreme Court this fall. Both involved government officials blocking members of the public on social media, but each led to a different result. The 9th Circuit found impermissible censorship, while the 6th Circuit did not. Due to the complexity of internet law, it’s unlikely Americans will feel good about the rule of law that will be articulated by the Supreme Court,” he said.
Still, if the NIH is “selectively listening to people online, then that’s hugely problematic,” Goldman said. In particular, the NIH could be denying the patients’ ability to learn and talk with each other. “Selective intervention by the NIH takes away that potential,” he said.
Advocates say the censorship has further eroded trust between the Long COVID community and the NIH. “It’s not just a one off,” Billy Hanlon, the director of advocacy and outreach for the Minnesota ME/CFS Alliance said, “It’s a pattern.” The agency fails to value the lived experience of patients with infection-associated illnesses, even though these illnesses have a quality of life worse than some advanced-stage cancers, Hanlon said.
“I can see why people were furious,” said JD Davids about the censorship “It’s an insult upon injury.” As the co-director of the advocacy group Long COVID Justice, Davids said that if the NIH wants to truly work and engage with patients, they need to work closely with people living with Long COVID and certainly not silence their lived experience.
“We need a government-wide response to Long COVID,” he said, describing the necessity for patients and complex chronic disease experts to be consulted on major decisions at the NIH and beyond. Tweeting that there is a treatment plan for a condition with no treatments or cures, Davids said, creates an illusion of a broader treatment plan for Long COVID, when there isn’t one. It confuses the public and creates doubt about people living with Long COVID. “It has huge unintended consequences,” Davids said.
Editor’s note: JD Davids has donated to the COVID-19 Data Dispatch. This had no influence on the article, as the author talked to him before the CDD decided to publish it.
Miles W. Griffis is an independent journalist based in Los Angeles, California. He’s written for High Country News, National Geographic, The New York Times, and many others.
If you are able to contribute a tip for this reporting, please Venmo @miles-griffis.
-
Sources and updates, August 13
- CDC identifies continued Long COVID risk: A new study from the CDC this week, published in the agency’s Morbidity and Mortality Weekly Report, summarizes data from the CDC and Census Household Pulse Survey examining Long COVID prevalence in the U.S. According to the survey, Long COVID prevalence declined slightly from summer 2022 to early 2023, but has remained consistent this year at about 6% of all U.S. adults. The survey also found that about one in four adults with Long COVID consistently report “significant activity limitations” from the condition, meaning they are less able to work and participate in other aspects of daily life. Treating Long COVID and supporting long-haulers should be priorities for the healthcare system, the study’s authors write.
- Mitochondrial dysfunction in Long COVID: Another new paper, published this week in Science Translational Medicine, demonstrates the role that mitochondria may play in Long COVID. Researchers at the Children’s Hospital of Philadelphia studied tissue samples from autopsies and animals infected with COVID-19, finding that the coronavirus led to malfunctioning mitochondria in several key organ systems. These malfunctions may contribute to Long COVID symptoms such as fatigue and brain fog, and could be a target for future treatments. Elizabeth Cooney at STAT News covered the study in more detail.
- Benefits of vaccination during pregnancy: One more notable new study: researchers at the National Institute of Allergy and Infectious Diseases (or NIAID, part of the NIH) tracked the impacts of COVID-19 vaccination for pregnant people. The study included 240 vaccinated participants who contributed blood samples, between July 2021 through January 2022. Both the parents and their newborns developed antibodies against the coronvirus following infection, the researchers found. While previous papers have demonstrated the value of vaccination for new parents, this study is one of the largest so far to show that protection is conferred to newborns.
- Wastewater surveillance webinar from the People’s CDC: If you’ve been following wastewater data to keep up with COVID-19 trends but have had questions about how this form of surveillance works, you may find it helpful to watch this recorded webinar from health advocacy organization the People’s CDC. In the video, Marc Johnson, a professor at the University of Missouri and director of the state’s wastewater surveillance program, talks through how wastewater is tested for the coronavirus (and variants), how to interpret wastewater data, cryptic lineages, and more. Understanding this novel data source is increasingly important now, as traditional healthcare data on COVID-19 are less reliable.
- New federal heat surveillance dashboard: Finally, in other public health news, the federal government has launched a new dashboard to track heat-related health issues. The dashboard compiles data from Emergency Medical Services reports across the country, representing responses to 911 calls for any health reason related to heat stress. (You can see the list of potential health events in the dashboard’s documentation.) Currently, many southern states are experiencing high levels of heat-related health problems, according to the dashboard. Many of the same states are also experiencing COVID-19 upticks right now—trends that may be related, as more people gather inside during hot weather.
-
NIH RECOVER’s Long COVID trials unlikely to lead to successful treatments, experts say
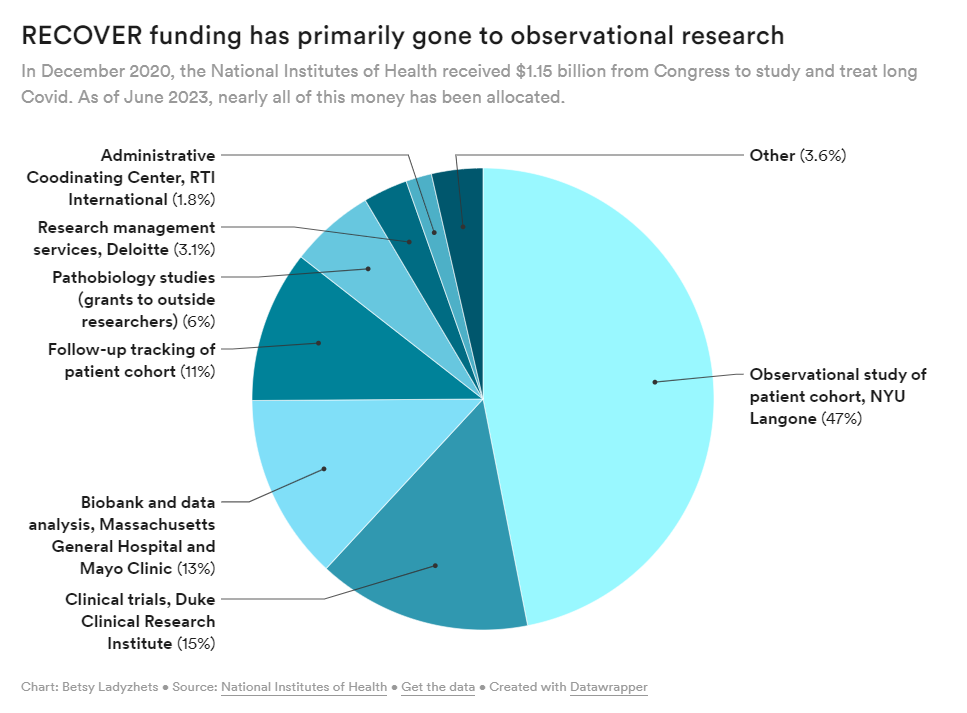
The NIH has primarily spent its funds for Long COVID research on observational studies rather than clinical trials, according to new data shared with my article this week. Last week, the National Institutes of Health and Duke University announced five Long COVID clinical trials as part of the NIH’s RECOVER initiative. This might sound like an exciting milestone for the millions of people dealing with long-term symptoms—but in fact, experts and long-haulers are disappointed by the trials, I learned when covering this news for MuckRock and STAT News.
RECOVER is the largest Long COVID research effort in the world; the NIH received $1.15 billion for it, provided by Congress in late 2020. It’s also been plagued by delays and criticisms, as I’ve reported before. As we approach the three-year mark of the initial funding, long-haulers are becoming increasingly frustrated with RECOVER’s lack of results.
My latest story for MuckRock and STAT focuses on the clinical trials, but connects to larger issues with RECOVER and with the federal government’s response to Long COVID in general. Read it on STAT’s site here or on MuckRock’s here.
A few key points from the story:
- RECOVER is only testing a handful of drugs for Long COVID, instead focusing on behavioral interventions that outside experts say are unlikely to address underlying causes of symptoms. There are several lists of potential drugs that should be (and aren’t) prioritized, including one compiled by members of an advisory committee to RECOVER.
- Looking more closely at the drug trials, experts shared concerns about the study designs, suggesting that RECOVER’s choices of controls, outcomes measures, and other aspects of the studies may lead to inaccurate results. For example, dysautonomia expert Lauren Stiles told me that the trial testing drugs for autonomic symptoms may fail to accurately capture whether those drugs help with Long COVID.
- At this point, the NIH has no plans for further Long COVID trials or other research going beyond RECOVER. The initiative has almost fully allocated all of its $1.15 billion in funding, and NIH officials haven’t shared details about how they will continue Long COVID research after this study concludes (though they acknowledge more research will be necessary).
RECOVER failed to put much funding in clinical trials to begin with, focusing instead on observational studies aiming to track Long COVID symptoms over time. While such studies could be valuable for better understanding the condition, RECOVER has largely replicated other research and hasn’t contributed useful, new information to the field, experts have told me. In fact, over 40,000 people have petitioned the NIH to retract RECOVER’s first paper based on its observational research.
Many of RECOVER’s errors, such as choosing the wrong treatments to prioritize and focusing on observational studies over clinical trials, could’ve been avoided if the initiative had listened more to long-haulers and learned from experts in other post-infectious diseases. Long-haulers have done plenty of research themselves in the last three years, ranging from informal tests of different treatments to formal studies conducted by the Patient-Led Research Collaborative; yet these studies have not informed RECOVER.
Plus, scientists with expertise in ME/CFS, dysautonomia, HIV/AIDS, and many other similar diseases could share lessons with RECOVER—but they aren’t leading the initiative. I thought Todd Davenport, a rehabilitation expert at University of the Pacific who’s studied ME/CFS, put it well when he said that RECOVER scientists “have parachuted into post-infectious illness and are now trying these things for the first time, to them. But it’s clear they haven’t done the reading.”
I hope to continue covering RECOVER and other issues with Long COVID research in the U.S. If you have any tips or stories to share with me on this topic, please reach out.
-
COVID source shout-out: Ed Yong’s Long COVID coverage
Ed Yong, a widely-admired science journalist, recently announced that he’s leaving his position at The Atlantic after eight years at the publication. He also published the latest in a series of articles explaining the challenges of Long COVID, a subject that he’s become well-known for covering.
I have been a big fan of Yong’s for a long time; reading his work when I was in college was one of my inspirations for getting into science writing. But his COVID-19 coverage has been especially informative and inspirational. In particular, he was one of the first journalists to write about Long COVID back in 2020 and has remained a leading writer on the topic since then. His work has brought wider recognition to the long-haulers seeking research and support.
His latest story, like his others, is a master class in weaving together patient experiences and scientific insights. It covers fatigue and post-exertional malaise, two of the most common—and most debilitating—symptoms of Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). As people with those conditions have shared the article on social media, I’ve seen many say that it offers poignant descriptions of these symptoms and insightful understanding of their experiences.
If you haven’t read this article yet, please check it out. (Feel free to email me if you have a hard time accessing it through the paywall.) And I think I can speak for many readers here when I express gratitude for Ed Yong’s COVID-19 work over the last three years, and excitement for what he’ll do next!
-
Sources and updates, July 30
- New papers show wastewater’s predictive value: This week, I noted three recently-published papers that all demonstrate wastewater surveillance’s value for flagging changes in COVID-19 trends before other metrics, such as hospitalizations. One paper, in Nature, found that wastewater trends preempted hospitalization trends by one to four weeks, in 150 U.S. counties. Another paper, in JAMA Network Open, found that wastewater trends tracked with case trends in 268 U.S. counties from January through September 2022; however, the correlation became weaker with reduced clinical testing over time. And a third paper, in PNAS, shares an algorithm that can flag community-level COVID-19 surges before they show up in other metrics, using data from North Carolina’s wastewater surveillance.
- Long COVID’s impact on employment: The Urban Institute, a think tank focused on economic and social policy research, published a report exploring employment challenges and related hardships among people with Long COVID. The researchers (a group that included Lisa McCorkell from the Patient-Led Research Collaborative) analyzed results from Long COVID questions included in an Urban Institute survey, conducted among more than 7,500 American adults across the country in December 2022. Among the findings: 10% of adults with Long COVID stopped working for a period due to their symptoms while another 5% reduced their work hours; 24% limited activities outside of work; 42% reported food insecurity in the last year; 20% reported difficulty paying their rent or mortgage.
- Characterizing potential Long COVID phenotypes: Another Long COVID study from this week, published in The Lancet: a research consortium including several medical centers across Europe tracked patients over time, seeking to better understand different subtypes of the condition. The study included about 1,000 people with at least one Long COVID symptom, tracked over one year from their initial COVID-19 diagnosis. Researchers found four potential subtypes: one similar to ME/CFS (including fatigue and cognitive symptoms), one with respiratory symptoms, one with chronic pain, and one with changes to taste and smell. The researchers also noted some patient characteristics and aspects of acute illness that may contribute to increased risk of different subtypes.
- Outdoor transmission at a night market: One more notable new paper: researchers at local health agencies in China’s Zhejiang province reported on coronavirus transmission at an outdoor night market, in Frontiers in Public Health. In one day at the night market, three infected visitors led to 131 secondary cases, the researchers found. Based on samples from both people at the market and surfaces, the researchers estimated that particles of an Omicron BA.5 strain could linger for over an hour and still be contagious. The study suggests that, even in outdoor settings, transmission is still possible when other precautions aren’t taken.
- Acute Hospital at Home data: The Data Liberation Project, which collects and shares data from public records requests, recently published a dataset from a COVID-era program by the Centers for Medicare and Medicaid Services (CMS) which allowed hospitals to treat patients in their homes. Early this year, the project filed a FOIA request for data indicating which hospitals applied to participate in the program and how their patients fared. CMS completed the request in June, and DLP is working to process and understand the resulting data. If you’re interested in using the data, you can check out the documentation and sign up for updates.
- Diagnosis challenges with alpha-gal syndrome: Finally, a bit of non-COVID public health news: the CDC recently released some data showing challenges with diagnosing alpha-gal syndrome—a disease transmitted by tick bites that leads to new allergies—despite recent increases in its spread. The CDC estimates that up to 450,000 people in the U.S. may have been impacted by this disease, potentially developing new allergies to meat and other animal products. Yet in one CDC study, the majority of health providers surveyed were not confident in their ability to diagnose the syndrome. This trend reflects similar challenges for other chronic diseases that might be new or unfamiliar to providers, such as Long COVID.