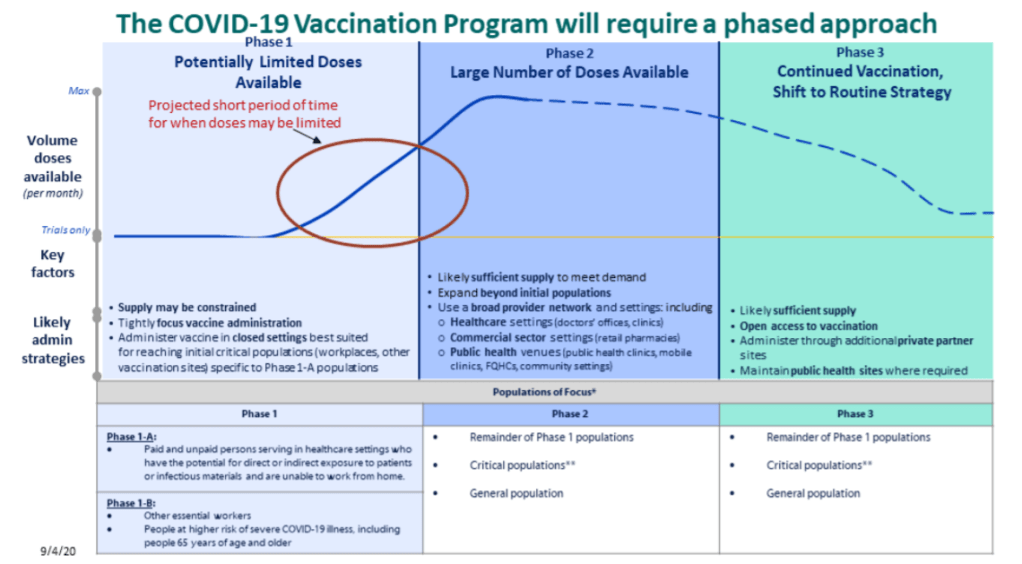
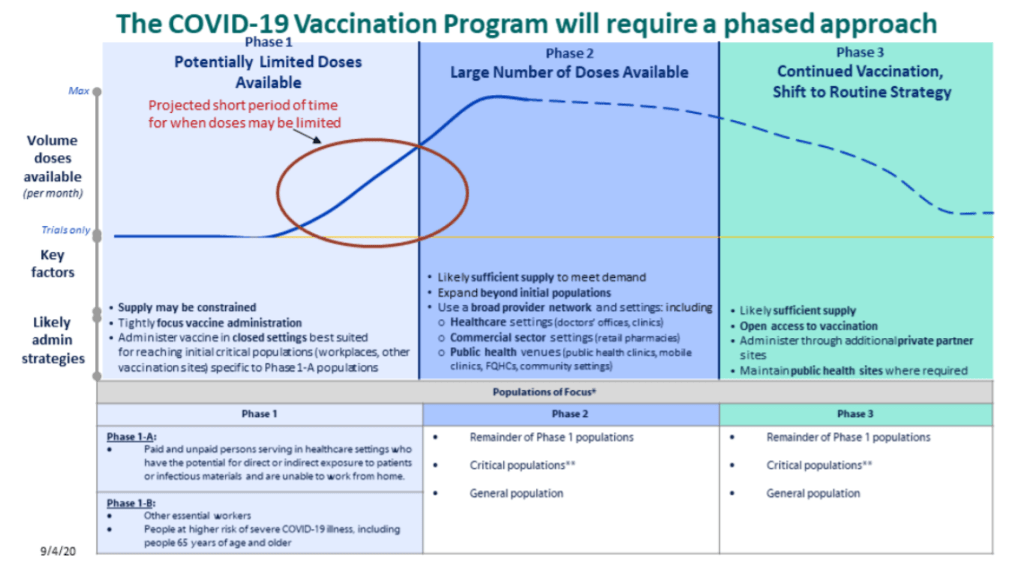
This past Friday, the Food & Drug Administration (FDA) officially issued Emergency Use Authorization for America’s first COVID-19 vaccine. This is a monumental achievement. One year ago, no Americans had even heard of the novel coronavirus; now, the federal government is beginning to ship out vaccine doses for our frontline healthcare workers.
But as excitement builds, so do concerns about the nation’s capacity to deploy vaccine doses to all who need them. The Atlantic’s Sarah Zhang wrote this week that we are entering a phase of “vaccine purgatory,” in which a myriad of challenges could delay the country’s path to herd immunity. Already, four states are claiming that they will be unable to start administering vaccinations until January, while several other states have deferred the decision about who gets a shot first to healthcare providers. While the CDC has issued guidances, many logistics are left up to states—the same type of fractured system which has prevented America from getting its testing under control.
True to my beat, I am most concerned about vaccine data. Earlier this week, the New York Times’s Sheryl Gay Stolberg reported that some states are refusing to report vaccination data to the CDC.
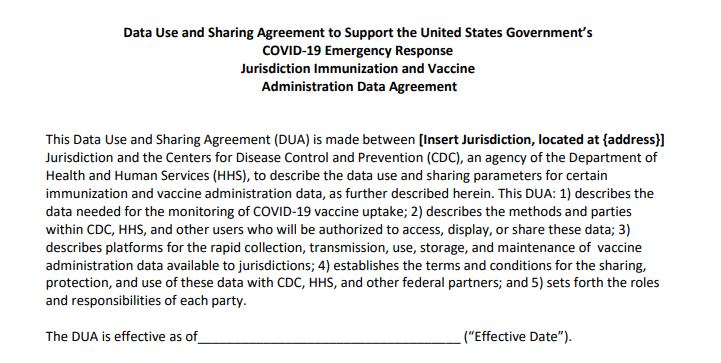
Historically, each state has tracked vaccinations independently, through Immunization Information Systems (or IIS). While the CDC does report some national immunization information, such as its flu vaccination dashboard, this information typically comes from outside surveys and is not reported in real-time. Of course, this won’t do for COVID-19. To build up a national system, the CDC has asked every state to sign a Data Use and Sharing Agreement promising to send vaccination counts and associated demographic data to the CDC.
It seems simple, right? But according to Stolberg’s reporting, the CDC has asked states to send personal information—such as names, birth dates, ethnicities, and home addresses—for each vaccine recipient. While demographic information should be tracked for COVID-19 vaccines in order to monitor equity in distribution, there is no need for the CDC to collect such specific information as names or home addresses. In fact, such a practice both discourages people from getting vaccinated and discourages states from cooperating with the federal public health agency.
Stolberg quotes an official from Minnesota who is concerned about privacy:
In Minnesota, officials are refusing to report any identifying details to the C.D.C., but they will submit “de-identified doses-administered data” on a daily basis once the vaccine campaigns begin.
“This is a new activity for us, as we don’t typically report this level of detail on this frequency to the federal government,” Doug Schultz, a spokesman for the Minnesota Department of Health, said in an email. He added, “We will not be reporting name, ZIP code, race, ethnicity or address.”
States which refuse to send personal data to the federal government may still report anonymous demographic information, such as the races and ethnicities of individuals who get vaccinated, on state-level dashboards. But it seems increasingly likely that vaccination data will face the same challenges as testing data: with every state deciding on a different reporting practice, it could be difficult to standardize and answer basic questions at the national level.
Other vaccine data news and resources from this week:
- USA Today has compiled every state’s COVID-19 vaccine distribution plan.
- Full scientific data from the Pfizer/BioNTech vaccine trial were released, while Johnson & Johnson reduced the size of its clinical trial (from 60,000 to 40,000 participants) thanks to COVID-19’s current rapid spread in the U.S.
- The Kaiser Family Foundation estimated the priority population for vaccination in every state (a.k.a. healthcare workers with direct patient contact and residents of long-term care facilities).
- WalletHub ranked the cities which will need the most initial vaccinations, based on their shares of healthcare workers, seniors, and residents with critical health conditions that constitute COVID-19 risk.
- The CDC has developed a new smartphone app called v-safe (yes, in all lowercase), which will use texts and surveys to check in on vaccine recipients after they get their shots. The app will be optional, but CDC officials are hoping it can be a crucial piece of vaccine safety monitoring.
- Google News is teaming up with the Australian Science Media Centre to build a COVID-19 Vaccine Media Hub and support fact-checking research. The hub will provide research updates and access to scientific expertise; it will first launch in the U.K., then become available in other countries.