We now have two new COVID-19 vaccines available for this year’s respiratory virus season, one from Pfizer and one from Moderna, which are expected to perform well against current variants. The FDA approved both vaccines this week, and the CDC recommended them for almost all Americans. A third option, from Novavax, may become available in the coming weeks as well.
The federal government aims to present this fall’s shots as the next iteration in routine, annual COVID-19 vaccines—similar to the routine we’re all used to for flu shots. In fact, I’ve seen some news suggesting that the federal health agencies don’t want us to call these shots “boosters,” instead calling them “updated” shots or annual shots.
But this fall’s vaccine rollout is likely to be anything but routine, as it’s the first rollout following the end of the federal COVID-19 public health emergencies. The government is no longer purchasing shots and distributing them for free; now, insurance companies will have to cover the shots.
As a result, many Americans—especially those without health insurance—will have a harder time accessing these vaccines than they have for previous shots. Plus, the federal emergency’s end will make it harder for us to track how the vaccines are performing, as the coronavirus continues to evolve into new variants.
With all of these complications in mind, here are ten key facts and statistics that you should know about this fall’s COVID-19 vaccines.
Pfizer and Moderna’s shots have been approved and recommended for all Americans, ages six months and older.
Despite some debates among scientists about whether younger people really need updated COVID-19 shots, the FDA has approved these vaccines—and the CDC has recommended them— for all age groups. This is important because CDC recommendations are often the basis for insurance coverage, as experts explained at a webinar hosted by the National Press Foundation on Tuesday.
The shots exclusively target XBB.1.5, a coronavirus lineage that is common in the U.S. and globally right now.
According to the CDC’s genomic surveillance program, almost all cases in the U.S. in recent weeks have been caused by XBB.1.5 or related variants from the XBB lineage. Variants like EG.5 and FL.1.5.1 are also XBB descendants, which have been given nicknames to make it a bit easier for scientists to keep track of them.
It’s also important to note that, unlike last year’s boosters, this fall’s shots are monovalent vaccines—meaning they only target XBB.1.5. The shots no longer target the original strain of SARS-CoV-2 that first circulated in 2020. Scientists generally approve of this choice, as the virus has mutated so much since that time.
Moderna’s booster led to a 17-fold increase in antibodies against XBB.1.5 and XBB.1.6.
The vaccine companies presented data to the CDC’s vaccine advisory committee on Tuesday. Moderna’s presentation included results from a study testing its new vaccine against several different variants, using blood samples from people who received the booster.
About one month after vaccination with Moderna’s booster, the participants had about 17.5 times more neutralizing antibodies against XBB.1.5, 16.7 times more against XBB.1.6, 14 times more against EG.5.1, and 10 times more against BA.2.86. Pfizer also presented data, suggesting that their vaccine should similarly perform well against current variants.
The new vaccines should lead to similar side effects as we’re used to from past mRNA shots.
Based on data that the vaccine companies presented to the CDC’s committee, this fall’s Pfizer and Moderna vaccines should lead to similar side effects—headache, fatigue, muscle pain, etc.—as many of us have expected from past rounds of COVID-19 shots. The companies, along with the CDC and FDA, will continue to monitor these vaccines for any safety issues that may emerge as people start to get them.
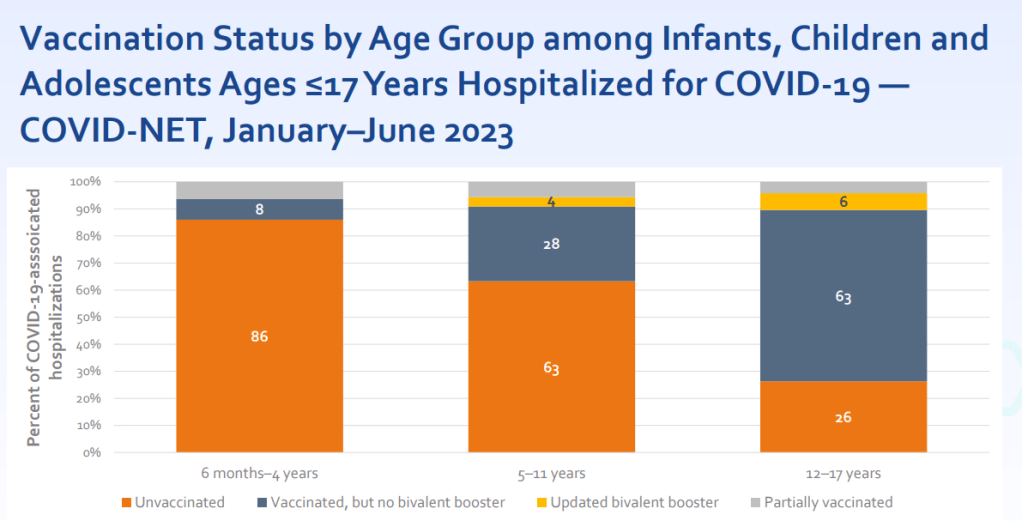
Young, unvaccinated children are at higher risk for COVID-19.
One of the CDC presentations focused on how this fall’s vaccines may benefit young children. Last fall and winter, hospitalization rates were higher for COVID-19 than for the seasonal flu across all young age groups, from infants (under six months) to 12-17 years old. The vast majority of the children hospialized were not vaccinated or hadn’t received last year’s booster.
For some CDC advisory committee members, these data were convincing in suggesting that this fall’s vaccine should be recommended for children, experts told STAT News. Vaccines updated to match current variants have a clear benefit for all age groups.
Long COVID remains a significant risk for Americans across age groups.
Another CDC presentation discussed Long COVID, as one of the potential adverse outcomes of a COVID-19 case. The CDC shared new data from a national survey conducted in 2022, which suggests that 9% of Americans ages 35 to 49 have experienced Long COVID symptoms (defined as symptoms lasting at least three months after a COVID-19 case). Adults ages 50-64 and 18-34 also reported high levels of Long COVID, at 7.4% and 6.8% ever experiencing symptoms, respectively.
Many studies have shown that vaccination lowers risk of Long COVID, though it does not by any means eliminate this risk. While it’s good to see the CDC incorporating Long COVID into its vaccine risk/benefit discussions, much more research is needed to better understand how to prevent this debilitating condition.
A Novavax vaccine is still in the pipeline.
Novavax also presented data to the CDC’s advisors this week, suggesting that its vaccine (also based on XBB.1.5) should perform similarly to the Pfizer and Moderna options. But unlike the Pfizer and Moderna vaccines, Novavax’s has yet to receive FDA approval. The company has said it’s still planning to distribute its vaccine this fall, but it’s unclear when the FDA may authorize it.
Some people are eager to receive the Novavax vaccine this fall, rather than Pfizer or Moderna’s, because this vaccine uses a different mechanism to boost the immune system. It may also lead to fewer side effects than the mRNA vaccine, making it a potentially good option for people who’ve had particularly strong reactions. (I know a couple of readers have sent me questions about this, and aim to do a deep-dive on Novavax in a future issue.)
Only 17% of Americans received last fall’s bivalent booster.
The booster uptake last year was low, according to the CDC. Even among seniors, only 43% received the booster. Can we do better this year?
A POLITICO/Morning Consult poll found that about 60% of respondents said they “probably or definitely” would get this year’s vaccine. (The poll included about 2,000 registered voters from across the U.S.) But it’s likely that access issues could get in the way for many people, as getting this COVID-19 vaccine will be much more challenging than it’s been in past rollouts.
HHS program should provide free vaccines for 25-30 million adults.
The Department of Health and Human Services has officially launched its “Bridge to Access” program, designed to provide free COVID-19 shots to uninsured Americans. Through this program, the HHS is essentially buying a small number of shots and distributing them to pharmacies, federally supported health centers, and other providers. You should be able to view these providers at vaccines.gov, according to the HHS. But I’ll be curious to see how well that actually works.
This year’s vaccine rollout will be much harder to track.
In the past, I’ve written about how the U.S. has failed to monitor breakthrough cases, or COVID-19 infections that occur after someone is vaccinated (and the hospitalizations, deaths, and long-term symptoms that may result). This year, not only are we failing to track breakthrough cases—the U.S. no longer has any national case data at all. We also no longer have vaccination data, as the CDC is not collecting this information from state and local health systems.
So, how will we know how this year’s vaccine rollout goes? It’ll likely be a lot of guesswork, extrapolating from a few state/local health departments, polling data, and other smaller-scale research to estimate how many people are getting vaccinated nationally. This challenge is just another example of the damage that the federal government has done in the last year by dismantling many of its COVID-19 data systems.