- New respiratory virus dashboard for Europe: Residents of about 50 European countries can now follow respiratory virus trends for their nations on a new dashboard developed by the World Health Organization (WHO) and European Centre for Disease Prevention and Control (ECDC). The dashboard compiles data from patient visits to healthcare facilities, laboratory testing, and genetic sequencing of coronavirus variants, according to a press release by the ECDC. Viewers can find summary trends for influenza-like illness as well as specific trends for COVID-19, flu, and RSV. This dashboard is a great step forward for standardizing surveillance data across countries.
- Medicaid unwinding update from KFF: This week, the Kaiser Family Foundation (KFF) published an update to its Medicaid Enrollment and Unwinding Tracker, which follows the Americans who lost their health insurance following the end of a Medicaid rule tied to the federal public health emergency for COVID-19. At least 10 million people have lost Medicaid coverage as of November 1, KFF reports, though the researchers acknowledge that this number is likely an undercount due to limited data. While disenrollment rates vary by state, overall, 71% of people who lost Medicaid coverage did so for “procedural reasons,” i.e. paperwork issues, according to KFF’s analysis.
- New Long COVID prevalence estimates: In a new paper, published this week in PLOS ONE, researchers at Dartmouth and University College London estimate Long COVID prevalence in the U.S. based on six months of data from the U.S. Census and CDC’s Household Pulse Survey. (Longtime readers may remember that this survey is one of my personal favorite sources for Long COVID data.) About 14% of respondents surveyed between June and December 2022 reported that they had experienced Long COVID at some point, half of them during the time they were surveyed, the researchers found. Americans with less education and lower incomes were more likely to report Long COVID symptoms, and the condition was correlated with physical and mental difficulties such as trouble dressing and bathing.
- Vaccine confidence falling in the U.S.: A new study from the Annenberg Public Policy Center at the University of Pennsylvania finds that vaccine confidence is declining for a variety of diseases, not just COVID-19. The researchers compared results from similar surveys conducted in October 2023 and in April 2021, both of which included about 1,600 people selected for a nationally representative sample. Confidence rates in COVID-19 vaccines dropped from 75% to 63%, while confidence rates that all vaccines approved in the U.S. are generally safe fell from 77% to 71%. At the same time, the researchers found that more survey respondents believed incorrect statements, such as that ivermectin was an effective treatment for COVID-19.
- Reasons for masking in Japan: One more study that caught my attention this week, on a more positive note: a researcher at Osaka University examined Japanese use of masks for COVID-19. Among participants in the researcher’s online surveys, the majority reported still wearing masks in June 2023, even though COVID-19 guidelines in Japan became less strict earlier this year. Social norms in Japan contribute to this behavior, the survey found, as respondents reported that they continued to mask both to avoid infection and to appear “normal” in public spaces. The study provides data behind well-known social norms in Japan, while offering some hope to those of us “lone maskers” in places where the norms are quite different.
Tag: vaccine hesitancy
-
Sources and updates, November 5
-
Sources and updates, October 1
- CDC publishes Long COVID data from national survey: Every year, the CDC conducts the National Health Interview Survey, a detailed look at population health in the U.S. through interviews of about 30,000 adults and 9,000 children. In 2022, the survey included questions about Long COVID, defining the condition as symptoms for at least three months after an initial COVID-19 case. This week, the CDC published data from the 2022 survey. Among the findings: about 6.9% of adults had ever experienced Long COVID, and 3.4% had it at the time of their interview. These figures were 1.3% and 0.5% for children, respectively. Women were more likely to experience it than men, and the survey identified other demographic differences (race, income, etc.). While many of the findings align with other Long COVID data, this CDC survey is unique in providing data on Long COVID in kids—which can be devastating for the small (yet significant) number of people impacted.
- Molnupiravir could lead to new coronavirus mutations: A new study, posted in Nature this week ahead of its final publication, identifies potential dangers of using the antiviral molnupiravir. (Molnupiravir, made by Merck, is a similar drug to Paxlovid but tends to be less effective, so it’s not used as widely.) For this study, researchers at the University of Cambridge, Imperial College London, and colleagues examined coronavirus sequence data and found that certain mutations were likely to increase after molnupiravir use. Researchers have already known that this antiviral could lead to more viral evolution, but the paper provides more details on specific mutation risks; further research may examine the drug’s implications for immunocompromised patients.
- Accessibility issues for COVID-19 websites: Many state and territorial COVID-19 websites don’t meet accessibility guidelines, making their key health information difficult for people who are blind or visually impaired to access, according to researchers at North Carolina State University. The researchers recently replicated a study that they’d first done in 2021, running checks on state sites against standard web accessibility guidance. “In 2021, none of these public-facing COVID-19 sites met all the checked WCAG guidelines, and things did not get any better in 2023,” study author Dylan Hewitt said in a statement. Issues include incompatibility with screen readers, limited color contrast, and no alt text for images.
- Polling data indicate higher interest in flu shots than COVID-19 shots: The Kaiser Family Foundation (KFF) has published a new round of polling data from its COVID-19 Vaccine Monitor, focusing on vaccinations this fall. About 58% of adults in the poll said they would get a flu shot this year, compared to 47% who said they would get an updated COVID-19 shot. Vaccine interest continues to be partisan, the poll suggested, with Democrats much more likely to express confidence in the updated COVID-19 vaccines’ safety than Republicans. Democrats were also more likely to respond to increased COVID-19 spread, with 58% of those polled saying they recently took more precautions in response to the surge this summer.
- New behavioral health survey data from the CDC: One more CDC update from this week: the agency has just published 2022 data from its Behavioral Risk Factor Surveillance System (BRFSS). The BRFSS involves interviews of more than 400,000 adults each year, including questions about alcohol use, tobacco use, immunizations, cancer screenings, mental health, and more. While the data aren’t directly related to COVID-19, this surveillance system may be a valuable source for reporters or researchers seeking contextual data about health behaviors in a particular state, city, or county.
-
Sources and updates, July 31
- KFF poll shows low vaccine uptake for young kids: This week, the Kaiser Family Foundation released an update from their COVID-19 Vaccine Monitor, an ongoing project tracking U.S. attitudes towards vaccines. This latest update focuses on children under age five, and the results are worrying: about 43% of parents with kids in this age group say they will “definitely not” get their child vaccinated, citing concerns about vaccine safety. Conservative parents and those who are unvaccinated themselves were particularly likely to be against vaccinating their young kids, KFF found.
- Vaccine side effects less common for second boosters: A new CDC study, published in this week’s Morbidity and Mortality Weekly Report, tracked reactions to COVID-19 boosters among Americans over age 50 using CDC monitoring systems. Among over 200,000 people who received third and fourth doses from the same vaccine manufacturer, side effects like a sore arm and fatigue were less common after the fourth dose compared to the third dose. Still, uptake for second boosters has been slow and potentially inequitable; the CDC recently published data on second boosters by race/ethnicity, showing that white Americans over age 50 are more likely to get this extra protection than non-white people in this age group.
- White House summit on next-generation COVID-19 vaccines: And one more piece of vaccine news for this week: the White House brought together federal officials, scientists, and pharmaceutical executives for a summit discussing next-generation COVID-19 vaccines. The summit highlighted vaccine candidates designed to work against many potential coronavirus variants, as well as those that would be delivered through the nose—potentially producing more protection against coronavirus infection and transmission. Either option would require a lot of funding from a Congress that has been hesitant to support COVID-19 efforts.
- States are letting health emergency declarations expire: While the federal declaration of COVID-19 as a public health emergency will remain in place at least through this fall, many states have let their declarations expire in recent months. These expirations impact the resources states are able to allocate for tracking and responding to COVID-19—ranging from data collection to telehealth access. The ending emergencies are certainly contributing to less frequent COVID-19 data updates in many states.
- New studies on COVID-19’s origins: Two major studies have conclusively linked the coronavirus’ early spread to the Huanan Seafood Market in Wuhan, China. These studies, both published in Science, were produced by an international group of virologists and evolutionary biologists at the Scripps Research Institute, the University of Arizona, the University of Sydney, the University of Edinburgh, and many other institutions. The experts traced early cases in the seafood market, finding evidence of spillover from animals to humans. The precise origins of COVID-19 are still unknown, but these studies go a long way in demonstrating early spread tied to animals, not a lab leak.
-
Sources and updates, March 6
A couple of data sources, a couple of data-related updates:
- State plans for utilizing COVID-19 relief funding: The federal Office of Elementary and Secondary Education has posted every state’s plan for utilizing ESSER funding, a $13-billion fund set aside to help schools address the impact of COVID-19. Money can be utilized for academic assistance, improving ventilation in schools, testing, and more. State plans were due to the federal government last June, though some materials are still pending on the website.
- New GAO report on Long COVID: Between 8 and 23 million Americans may have developed Long COVID in the last two years—and an estimated one million are out of work because of this condition—according to a new report from the U.S. Government Accountability Office. The report discusses medical and economic impacts of Long COVID, including current efforts by the federal government to study the condition.
- KFF COVID-19 Vaccine Monitor update: This week, the Kaiser Family Foundation published a new report detailing America’s sentiments on COVID-19 vaccines and other pandemic issues. Key findings include: COVID-19 vaccine uptake “remains relatively unchanged since January” for both adults and children; a majority of parents with children under five say they “don’t have enough information” about vaccines for that age group; and “most adults believe that the worst of the COVID-19 pandemic is over but there are disagreements about what returning to normal means and when it should happen.”
- Vaccination disparities between urban and rural counties: Here’s a CDC MMWR study that caught my eye this week: researchers compared vaccination rates in urban and rural U.S. counties, finding that the rate of people in urban counties who have received at least one dose (75.4%) is much higher than the rate in rural counties (58.5%). Moreover, the gap between urban and rural counties has more than doubled between April 2021 and January 2022, the researchers found.
- CDC updates seroprevalence data: The CDC recently updated a dashboard showing data from seroprevalence surveys, which use information from labs across the country to estimate how many Americans have resolving or recent coronavirus infections. (This does not include vaccinations, unlike other seroprevalence estimates.) According to this new update, about 43% of the country had antibodies from a recent infection as of late January. In some parts of the country that were harder-hit by Omicron, the esimate is over 50%.
-
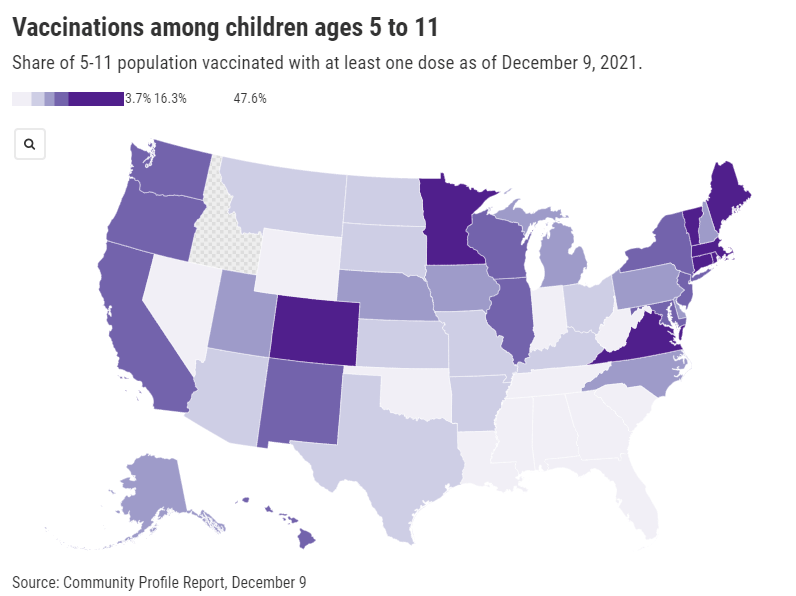
One month into vaccinations for kids 5-11, uptake varies wildly by state
It’s been about a month since the FDA and CDC authorized a version of Pfizer’s vaccine for children ages five to 11. Those kids whose parents immediately took them to get vaccinated are now eligible for their second doses, and will be considered fully vaccinated by Christmas.
Despite widespread availability of the shots, vaccine uptake has varied wildly: the share of children ages five to 11 who have received at least one dose ranges from almost 50% in Vermont—to under 4% in West Virginia. In Idaho, so few children in this age range have received a vaccine dose that the CDC has yet to report a number of children vaccinated.
As you can see from the map (which uses data as of December 9), vaccination rates for kids are falling pretty much along partisan lines, with states in the Northeast and West Coast vaccinating more than those in the South and Midwest. This is unsurprising yet troubling, as the states with lower vaccination rates among kids are also those states with more lax COVID-19 safety measures in schools—suggesting that they’re exactly the kids who could use that protection.
A new report from the Kaiser Family Foundation’s COVID-19 Vaccine Monitor provides context on slowing vaccination rates among children. According to KFF’s polling, three in ten American parents—both of teenagers and younger kids—say they will “definitely not” get their children vaccinated. Concerns about safety and potential long-term side effects abound, even though all data so far have suggested that the vaccines are very safe for children.
While the overall data are troubling, we lack information in one key area: demographic data. Without breakdowns of child vaccination rates by race and ethnicity, it’s difficult to say whether the racial gap in vaccinations that we saw for adults earlier in 2021 has persisted for younger Americans. This data absence makes it difficult for policymakers and health advocates to address the potential need for vaccine messaging tailored to families of color.
More vaccination data
-
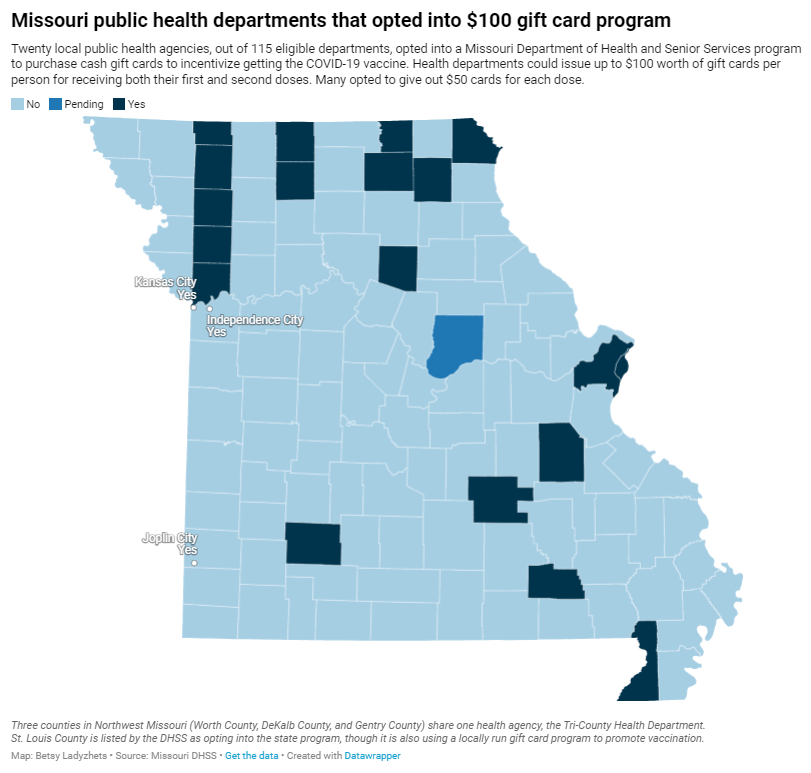
Cash incentives for vaccination have little impact
Over the past year, vaccine incentives have become a popular strategy among businesses and state and local governments. From free donuts to free Mets tickets, Americans have had opportunities to get bonus rewards along with protection from the coronavirus. And one particularly common incentive is cash, offered through small payments accompanying vaccinations and lotteries that only vaccinated people can enter.
While politicians at all levels have praised cash incentives, research has shown that this strategy has little impact on actually convincing Americans to get vaccinated. A recent investigation I worked on (at the Documenting COVID-19 project and the Missouri Independent) provides new evidence for this trend: the state of Missouri allocated $11 million for gift cards that residents could get upon receiving their first or second vaccine dose, but the vast majority of local health departments opted not to participate in the program—and a very small number of gift cards have been distributed thus far.
The Missouri program’s limited success fits into a national pattern. “It’s hard to tease out a causal effect of a program that’s not introduced with the purpose of a research experiment,” Dr. Allan Walkey, an epidemiologist at Boston University who’s studied vaccine incentives, told me. Still, Walkey said, the majority of research on these programs has found that cash incentives are not driving huge numbers of people to get their shots.
Walkey specifically studied a vaccine lottery in Ohio, the first state to set up such a program. While initial reports by state leaders suggested that a lot of people got vaccinated after the lottery was announced, Walkey found that, in fact, the new vaccinations were more likely caused by an expansion of vaccine eligibility. Two days before the lottery was announced, the Pfizer vaccine was authorized for children between the ages of 12 and 15.
The lottery “didn’t have a large effect on vaccine uptake,” Walkey told me. Studies of vaccine lotteries in other states have found similar results.
For this story, I also spoke to Ashley Kirzinger, a polling expert at the Kaiser Family Foundation (KFF) who helps run KFF’s Vaccine Monitor surveys. In these surveys, KFF sorts unvaccinated Americans into categories based on their vaccine attitudes: “wait and see,” “only if required,” and “definitely not.” Kirzinger told me that cash incentives, vaccine requirements for events, and other social pressures are more likely to “motivate the ‘wait and see’ or ‘only if required’” groups.
But for those Americans who “definitely” don’t want to get vaccinated, these incentives aren’t likely to move the needle. In fact, the people in this group may be angered by incentives, because they could see such programs as unfair pressure from the health system.
This was true in some Missouri local public health departments. For example, in Carter County—where the local agency did opt in to the state gift card program—a planned vaccination drive with the gift cards was canceled due to local opposition.
“So many parents and community members were upset, we were not allowed to hold the vaccination event at the school,” said Michelle Walker, the county health center administrator.
Overall, out of 115 local public health agencies in Missouri that were eligible to participate in the incentive program, just 20 opted to get gift cards. Most departments purchased $50 gift cards, so that residents could get $50 at their first vaccine dose and $50 at their second dose.
Through surveying the local agencies that participated, my colleague Tessa Weinberg and I obtained data from 10. Out of 6,378 gift cards that the agencies were able to purchase with state funding, we found that just 1,712 had been distributed so far, as of late November.
!function(){“use strict”;window.addEventListener(“message”,(function(e){if(void 0!==e.data[“datawrapper-height”]){var t=document.querySelectorAll(“iframe”);for(var a in e.data[“datawrapper-height”])for(var r=0;r<t.length;r++){if(t[r].contentWindow===e.source)t[r].style.height=e.data["datawrapper-height"][a]+"px"}}}))}();Read the full story for more on why many departments didn’t participate in this gift card program, and how it’s going for the departments that did opt in.
-
FDA authorizes Pfizer vaccine for younger children
The Pfizer vaccine will likely be available to children ages 5 to 11 next week, but many parents are hesitant about getting their kids vaccinated. Chart via the KFF COVID-19 Vaccine Monitor. Last week, the Food and Drug Administration (FDA) recommended Pfizer’s COVID-19 vaccine for children ages 5 to 11, under an Emergency Use Authorization. The agency’s vaccine advisory committee met on Tuesday to discuss Pfizer’s application and voted overwhelmingly in favor; the FDA followed this up with an EUA announcement on Friday.
This coming week, the process continues: CDC’s own vaccine advisory committee will discuss and vote on vaccinating kids in the 5-11 age group, and then the agency will make an official decision. If all goes well—and all is expected to go well—younger kids will be able to get their vaccines in time for Thanksgiving.
Many of the parents I know have been eagerly awaiting this authorization, but the sentiment is far from universal. COVID-19 vaccinations for kids are incredibly controversial, more so than vaccinations for adults. The public comment section of the FDA advisory committee meeting—in which basically anyone can apply to share their thoughts—was full of anti-vaxxers, many of them sharing misinformation. Even some experts on the FDA advisory committee were not fully convinced that vaccines are needed for all young kids, though all but one eventually voted in favor.
Now, let me be clear: there are definite benefits to vaccinating younger children. While kids are less likely to have severe COVID-19 cases than adults, the disease has still been devastating for many children. Almost 100 kids in the 5 to 11 age range have died of COVID-19, making this disease one of the top 10 causes of death for this group over the past year and a half.
Plus, children who get infected with the coronavirus are at risk for Long COVID and MIS-C, two conditions with long-lasting ramifications. There have been about 5,200 MIS-C cases thus far—and the majority of these cases have occurred in Black and Hispanic/Latino children. Minority children are also at much higher risk for COVID-19 hospitalization.
Vaccination can prevent children from severe ramifications of a potential COVID-19 case, as well as from the mild infections that lead to missed school and other disruptions. But the FDA committee had to carefully weigh this benefit against potential side effects from vaccination, namely myocarditis—a type of heart inflammation.
The U.S. system for tracking vaccine side effects has identified a small number of myocarditis cases in children ages 12 to 15 after their second shots of Pfizer or Moderna vaccines. For the meeting this past Tuesday, the FDA presented some models weighing potential myocarditis cases in young kids against vaccination benefits; the models showed that, in almost every scenario, the number of severe COVID-19 cases prevented by vaccination is higher than the myocarditis cases.
It’s worth noting: in Pfizer’s clinical trial for the 5 to 11 age group, no child had a severe adverse reaction to the vaccine. But the Pfizer researchers did observe five medical events that were unrelated to vaccination—including one kid who swallowed a penny.
Some of the FDA advisory committee members suggested that perhaps vaccines would be most beneficial for children with underlying medical conditions, who are more susceptible to severe COVID-19. But the committee ultimately voted in favor of vaccines for all kids in the 5 to 11 age group, allowing parents to consult their pediatricians and pursue vaccination if they deem it necessary.
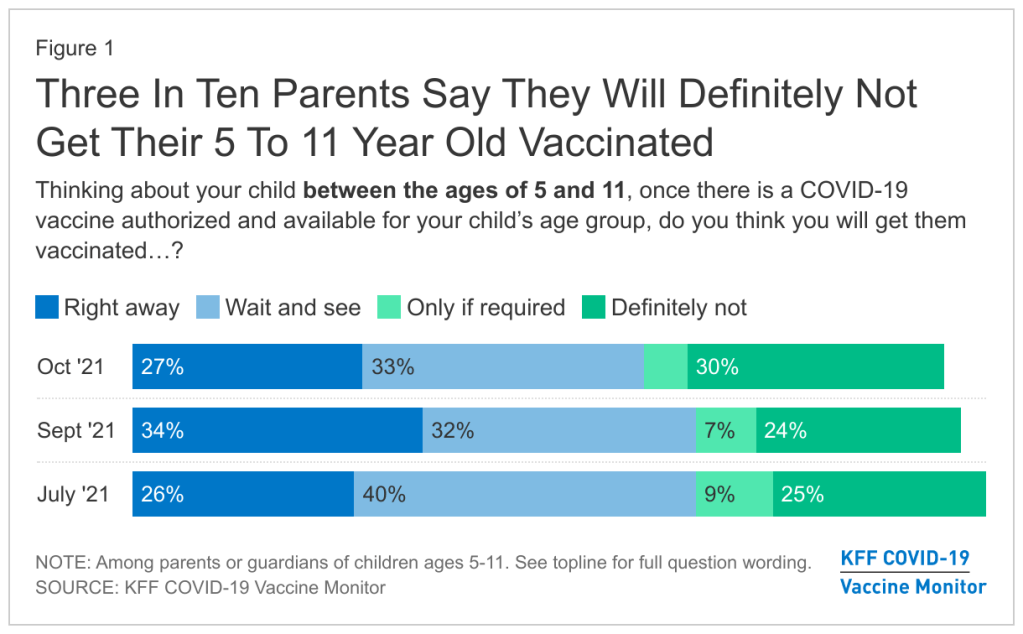
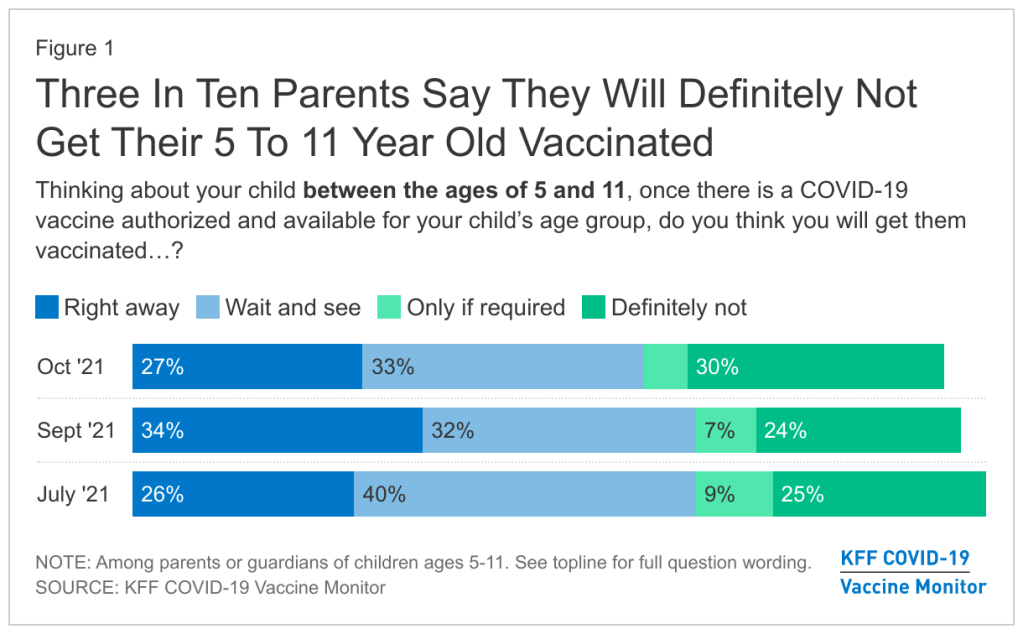
Polling data suggest that many parents don’t currently deem it necessary, though. The latest survey from the Kaiser Family Foundation found that just 27% of parents with kids in the 5 to 11 age range plan to get their kids vaccinated immediately, once shots are available. 33% intend to “wait and see,” 5% will only pursue vaccination if it’s required by the child’s school, and 30% say “definitely not.”
Public health experts, pediatricians, and others in the science communication world have a lot of work ahead of us to convey the importance of vaccinating kids—and dispel misinformation.
Note: this post relies heavily on STAT News’s liveblog of the FDA committee meeting.
More vaccine coverage
-
Featured sources, September 19
- COVID Behaviors Dashboard from Johns Hopkins: John Hopkins University maintains one of the oldest and best-known COVID-19 dashboards of the pandemic. The team recently expanded its data offerings with a new dashboard focused on pandemic attitudes and practices around the world. This dashboard draws from surveys conducted in over 100 countries, in collaboration with the WHO; read more about it here.
- COVID-19 K-12 School Testing Impact Estimator: What COVID-19 testing strategy would make the most sense for your local K-12 school? This dashboard, by the Rockefeller Foundation and Mathematica (the data research organization), is designed to help stakeholders find out. Simply plug in the school’s characteristics and COVID-19 safety goals, and the dashboard will tell you how different testing strategies may measure up.
- Vaccine hesitancy roundup from the Journalist’s Resource: This resource page includes a wealth of data and insights on vaccine hesitancy in the U.S., drawing from a variety of surveys and research papers on the topic. As of early September, author Naseem Miller writes, the PubMed research database included over 750 studies on COVID-19 vaccine hesitancy, signifying growing academic interest in this topic.
- Hospital challenges to public health reporting: A new report from the National Coordinator for Health Information Technology explores the challenges that non-government hospitals have faced in electronically exchanging information with public health agencies. One major finding: in both 2018 and 2019, half of all hospitals lacked the capacity for this data exchange. No wonder electronic reporting has been such a challenge during the pandemic.
- NIH Long COVID initiative revs up: This isn’t an actual data source, more of an update: the National Institutes of Health (NIH)’s RECOVER Initiative to study Long COVID awarded a major research grant this week. About $470 million goes to New York University’s Langone Medical Center, which will serve as a national hub for Long COVID research and award sub-grants to other institutions. The NIH’s RECOVER website currently reports that between 10% and 30% of people infected with the coronavirus will go on to develop Long COVID; hopefully research at NYU and elsewhere will lead to some more precise numbers.
-
Featured sources, July 18
- COVID-19 resources by Evidence Aid: Evidence Aid is a U.K.-based nonprofit that provides evidence-based guidance for disaster response. The organization’s COVID-19 page includes plain-language research summaries about COVID-19 epidemiology, treatments, and more, available in several different languages.
- Public Health England Technical Briefings on SARS-CoV-2 variants: While the CDC has not done the best job of providing data on variants and breakthrough cases, the U.K.’s public health agency is sequencing more cases than any other country—and providing detailed reports on the results of those efforts. These reports may be useful for anyone seeking to keep a close eye on Delta and other variants’ ability to beat our vaccines. (h/t Your Local Epidemiologist)
- Excess mortality and COVID-19 deaths in 67 countries: Researchers from the University of Bologna (in Italy) analyzed the gaps between excess deaths and COVID-19 deaths in 67 countries, revealing the capacity of different national health systems to accurately identify COVID-19 cases. Their work was published this week in JAMA Network Open. (For more on excess deaths, see this CDD post about Peru.)
- Characterizing long COVID in an international cohort: In another new paper, published this week in The Lancet, COVID-19 long-haulers from the Patient-Led Research Collaborative share the results of an international survey on long COVID-19. The findings indicate that the vast majority of long-haulers (over 90% of those surveyed) suffer from symptoms for at least 35 weeks.
- COVID-19 Vaccine Acceptance and Hesitancy in Low and Middle Income Countries: One more new paper, this one published in Nature: an international group of researchers analyzed vaccine acceptance across several low- and middle-income countries (LMICs), the U.S., and Russia. They found much higher vaccine acceptance in LMICs (80%) compared to the U.S. (65%) and Russian (30%). The study data are available on GitHub.
-
Featured sources, June 27
- Vaccine hesitancy by ZIP code: A new data visualization tool from the Institute for Health Metrics and Evaluation provides details on which parts of the U.S. would most benefit from vaccination campaigns. The underlying data come from a survey run by the Delphi Research Group at Carnegie Mellon, conducted between June 4 and June 10.
- OIG report on nursing homes: The HHS Office of Inspector General published a new report this week evaluating COVID-19 outbreaks in nursing homes. The report found that two in five Medicare beneficiaries living in nursing homes were diagnosed with COVID-19 (confirmed or probable cases) in 2020, and almost 1,000 more seniors died per day in April 2020 compared to April 2019.
- The State of the Nation’s Housing, 2021: This comprehensive report from the Joint Center for Housing Studies at Harvard provides data on home prices, rents, and other related metrics for the past year. The report shows that many households—especially those who are Black and Hispanic—are still behind on housing payments, and could benefit from continued assistance (such as the CDC eviction moratorium extended this week).