- CDC investigating deaths from Long COVID: Researchers at the CDC’s National Center for Health Statistics are currently working to investigate potential deaths from Long COVID, according to a report from POLITICO. The researchers are reviewing death certificates from 2020 and 2021, looking for causes of death that may indicate a patient died from Long COVID symptoms rather than during the acute stage of the disease. There’s currently no death code associated with Long COVID and diagnoses can be highly variable, so the work is preliminary, but I’m really looking forward to seeing their results.
- CDC reports on ventilation improvements in schools: And one notable CDC study that was published this week: researchers at the agency from COVID-19, occupational health, and other teams analyzed what K-12 public schools are doing to improve their ventilation. The report is based on a survey of 420 public schools in all 50 states and D.C., with results weighted to best represent all schools across the country. While a majority of schools have taken some measures to inspect their HVAC systems or increase ventilation by opening windows, holding activities outside, etc., only 39% of schools surveyed had actually replaced or upgraded their HVAC systems. A lot more work is needed in this area.
- Results from the COVID-19 U.S. State Policy database: The Boston University team behind the COVID-19 U.S. State Policy database has published a paper in BMC Public Health sharing major findings from their work. The database (which I’ve shared in the CDD before) documents what states have done to curb COVID-19 spread and address economic hardship during the pandemic, as well as how states report COVID-19 data. In their new paper, the BU team explains how this database may be used to analyze the impacts of these policy measures on public health.
- Promising news about Moderna’s bivalent vaccine: Moderna, like other vaccine companies, has been working on versions of its COVID-19 vaccine that can protect better against new variants like Omicron. This week, the company announced results (in a press release, as usual) from a trial of a bivalent vaccine, which includes both genetic elements of the original SARS-CoV-2 virus and of Omicron. The bivalent vaccine works much better than Moderna’s original vaccine at protecting against Omicron infection, Moderna said; still, scientists are skeptical about how the vaccine may fare against newer subvariants (BA.2.12.1, BA.4, BA.5).
- Call center and survey from FYLPRO: A reader who works at the Filipino Young Leaders Program (FYLPRO) requested that I share two resources from their organization. First, the program has set up a call center aimed at helping vulnerable community members with their COVID-19 questions. The call center is available on weekdays from 9 A.M. to 5 P.M. Pacific time in both English and Tagalog; while it’s geared towards the Filipino community, anyone can call in. And second, FYLPRO has launched a nationwide survey to study vaccine attitudes among Filipinos; learn more about it here.
Tag: Moderna
-
Sources and updates, June 12
-
Booster shots: What we’ve learned—and what we still don’t know
This week, the FDA’s vaccine advisory committee had a two-day meeting to discuss booster shots for Moderna’s and Johnson & Johnson’s COVID-19 vaccines. From the outside, these meetings may have appeared fairly straightforward: the committee voted unanimously to recommend booster shots for both vaccines.
But in fact, the discussions on both days were wide-reaching and full of questions, touching on the many continued gaps in our knowledge about the need for additional vaccine doses. The FDA committee continues to make decisions based on rather limited data, as do other top U.S. officials. Case in point: on Thursday, the committee was asked to consider data from Israel’s booster shot campaign—which is utilizing Pfizer vaccines—as evidence for Moderna boosters in the U.S.
In the Moderna vote on Thursday afternoon, committee member Dr. Patrick Moore, a virologist at the University of Pittsburgh, said that he voted “on gut feeling rather than really truly serious data.” The comment exemplified how much we still don’t know regarding the need for boosters, thanks in large part to the CDC’s failure to comprehensively track breakthrough cases in the U.S.
Still, there are a few major facts that we have learned since the FDA and CDC discussions on Pfizer boosters that took place a couple of weeks ago. Here’s my summary of what we’ve learned—and what we still don’t know.
What we’ve learned since the Pfizer discussion:
Israel’s booster rollout continues to align with falling case numbers. On Thursday, representatives from the Israeli national health agency presented data on their booster shot rollout—which, again, is using Pfizer vaccines. The vast majority of seniors in Israel have now received a third dose, and over 50% of other age groups have as well. According to the Israeli scientists, this booster rollout both decreased the risk of severe COVID-19 disease for older adults and helped to curb the country’s Delta-induced case wave, causing even unvaccinated adults to have a decreased risk of COVID-19.
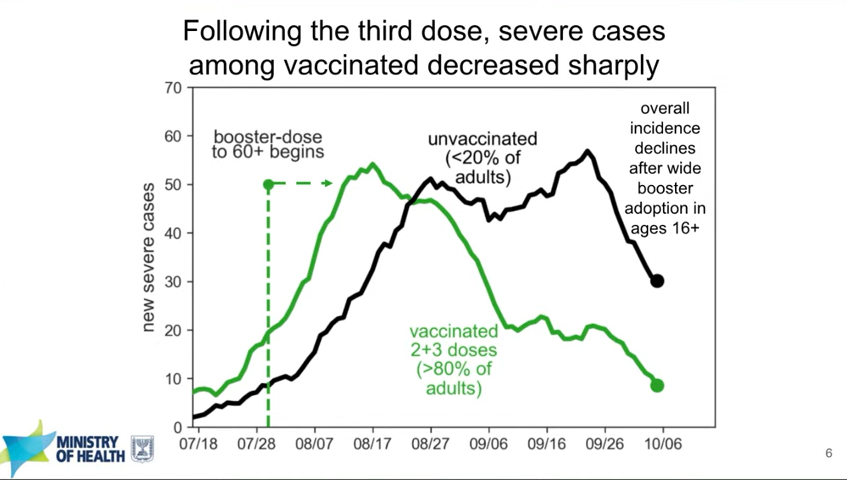
In Israel, severe cases among both vaccinated and unvaccinated adults decreased after the country provided third Pfizer doses to its residents. Screenshot taken from Thursday’s VRBPAC meeting. You can read more about Israel’s booster campaign in this paper, published in the New England Journal of Medicine in early October. It’s worth noting, however, that Delta is known to spur both case increases and decreases in cycles that can be somewhat unpredictable—and may not be exactly linked to vaccination. So, I personally take the Israeli claims that boosters stopped their case wave with a grain of salt.
Decreased vaccine effectiveness against infection may be tied more to Delta and behavioral factors than “waning antibodies.” This week, the New York State Department of Health (DOH) announced results from a large study of vaccine effectiveness which is, from what I’ve seen, the first of its kind in the U.S. The New York DOH used state databases on COVID-19 vaccinations, tests, and hospitalizations to examine vaccine effectiveness against both infection and hospitalization in summer 2021, when Delta spread rapidly through the state.
They found that vaccine effectiveness against infection did decline over the summer. But the declines occurred similarly for all age groups, vaccine types, and vaccine timing (i.e. which month the New Yorkers in the study received their vaccines)—suggesting that the decline in effectiveness was not tied to waning immune system protection. Rather, the effectiveness decline correlated well with Delta’s rise in the state. It also correlated with reduced safety behaviors, like the lifting of New York’s indoor mask mandate and the reopening of various businesses.
Vaccine effectiveness against hospitalization declined for older adults, but remained at very high levels for New Yorkers under age 65, the study found. Here’s what lead author Dr. Eli Rosenberg said in a statement:
The findings of our study support the need for boosters in older people in particular, and we encourage them to seek out a booster shot from their health care provider, pharmacy or mass vaccination site. We saw limited evidence of decline in effectiveness against severe disease for people ages 18 to 64 years old. While we did observe early declines in effectiveness against infections for this age group, this appears to have leveled off when the Delta variant became the predominant strain in New York. Together, this suggests that ongoing waning protection may be less of a current concern for adults younger than 65 years.
I was surprised that this study didn’t come up in the FDA advisory committee meetings this week, and will be curious to see if it’s cited in future booster shot discussions. The study does align, however, with the committee’s decision against recommending booster shots for all adults over age 18 who received Moderna vaccines.
Johnson & Johnson vaccine recipients appear to need boosters more than mRNA vaccine recipients. On Friday, presentations from both J&J representatives and FDA scientists made a clear case for giving J&J vaccine recipients a second dose of this adenovirus vaccine. In one 30,000-patient study, patients who received a second J&J shot two months after their first shot saw their vaccine efficacy (against symptomatic infection) rise from 74% to 94%.
Interestingly, unlike the Pfizer and Moderna vaccines, a J&J shot’s ability to protect against coronavirus infection appears relatively stable over time. However, a booster shot can make this vaccine more effective—especially against variants. Despite arguments from J&J representatives that their vaccine’s second dose should come six months after the first dose, the FDA advisory committee voted to recommend second J&J shots just two months after the first dose, for all adults over age 18.
It’s worth noting that this vaccine regimen might effectively change J&J’s product from a one-shot vaccine to a two-shot vaccine. STAT’s Helen Branswell and Matthew Herper go into the situation more in their liveblog.
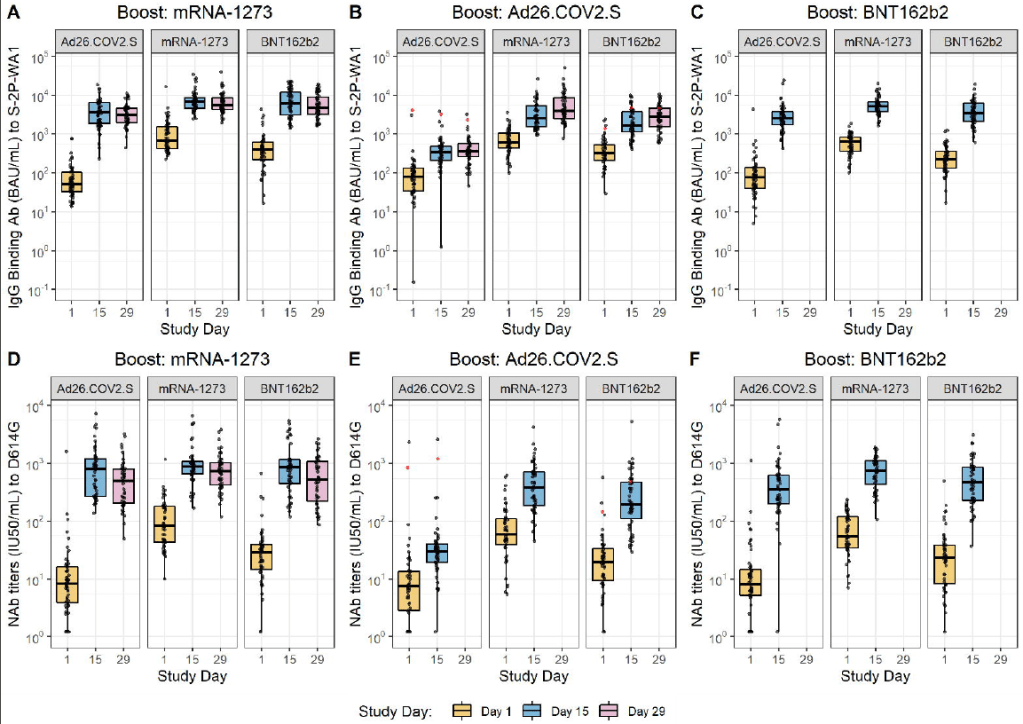
Mixing and matching vaccines is a strong strategy for boosting immunity, especially if one of the vaccines involved uses mRNA technology. This week, the National Institutes of Health (NIH) released a highly anticipated study (posted as a preprint) on mix-and-match vaccine regimens. The NIH researchers essentially tested every possible booster combination among the three vaccines that have been authorized in the U.S. Before and after vaccination, the researchers took blood samples and tested for antibodies that would protect against the coronavirus.
In short, the NIH study found that all three vaccines—Pfizer, Moderna, and J&J—will provide a clear antibody boost to people who have received any other vaccine. But the mRNA vaccines (Pfizer and Moderna) provide bigger benefits, both in the form of higher baseline antibody levels (after two shots) and a higher boost. The best combination was a J&J vaccine initially, followed by a Moderna booster, Dr. Katelyn Jetelina notes in a Your Local Epidemiologist summary of the study.

Every vaccine provided a “boost” of protective antibodies to recipients of every other vaccine. Figure from the NIH preprint. mrna-1273 refers to the Moderna vaccine, Ad26.COV2.S refers to the J&J vaccine, and BNT162b2 refers to the Pfizer vaccine. The booster regimens also appeared to be safe, with limited side effects. But this was a relatively small study, including about 450 people. In their discussion on Friday afternoon, the FDA advisory committee members said that they would be very likely to authorize mix-and-match vaccine regimens after seeing more safety data.
Moderna and J&J boosters appear to be safe, with similar side effects to second shots. Safety data from Moderna’s and J&J’s clinical trials of their booster shots, along with data from the NIH mix-and-match study, indicate that the additional doses cause similar side effects to first and second doses. After a booster, most recipients had a sore arm, fatigue, and other relatively minor side effects.
And here’s what we still don’t know:
Which medical conditions, occupations, and other settings confer higher breakthrough case risk? I wrote about this issue in detail in September. The U.S. continues to have little-to-no data on breakthrough case risk by specific population group, whether that’s groups of people with a specific medical condition or occupation. This data gap persists, even though U.S. researchers have some avenues for breakthrough risk analysis at their disposal (see: this post from last week).
This lack of data came up in FDA advisory committee discussions on Thursday. An FDA representative was unable to cite any evidence that people in specific occupational settings are at a higher risk for breakthrough cases.
Are there any rare vaccine side effects that may occur after breakthrough doses? When I covered the FDA advisory committee meeting on Pfizer boosters, I noted that Pfizer’s clinical trial of these shots included just 306 participants—providing the committee members with very limited data on rare adverse events, like myocarditis. Well, Moderna’s clinical trial of its booster shots was even smaller: just 171 people. J&J had a larger clinical trial, including over 9,000 people.
These trials and the NIH mix-and-match study indicated that booster shots cause similar side effects to first and second shots, as I noted above. But few clinical trials are large enough to catch very rare (yet more serious) side effects like myocarditis and blood clots. (In J&J’s case, blood clots occur roughly twice for every million doses administered.) Federal officials will carefully watch for any side effects that show up when the U.S.’s booster rollout begins for Moderna and J&J.
How do antibody levels correlate to protection against COVID-19, and what other aspects of the immune system are involved? The NIH mix-and-match study focused on measuring antibody levels in vaccine recipients’ blood, as did other booster shot trials. While it may sound impressive to say, for example, “J&J recipients had a 76-fold increase in neutralizing antibodies after receiving a Moderna booster,” we don’t actually know how this corresponds to protection against COVID-19 infection, severe disease, and death.
Some experts—including a couple of those on the FDA advisory committee—have said that discussions focusing on antibodies distract from other types of immunity, like the memory cells that retain information about a virus long after antibody levels have fallen. More research is needed to tie various immune system measurements to real-world protection against the coronavirus.
What needs to happen at the FDA for mix-and-match vaccination to be authorized? One challenge now facing the FDA is, the federal agency has clear evidence that mix-and-match vaccine regimens are effective—but it does not have a traditional regulatory pathway to follow in authorizing these regimens. Typically, a company applies for FDA authorization of its specific product. And right now, no vaccine company wants to apply for authorization of a regimen that would involve people getting a different product from the one that brings this company profit.
So, how will the FDA move forward? There are a couple of options, like the CDC approving mix-and-match boosters directly. See this article for more info.
Finally: I can’t end this post without acknowledging that, as we discuss booster shots in the U.S., millions of people in low-income countries have yet to even receive their first doses. Many countries in Africa have under 1% of their populations vaccinated, according to the Bloomberg tracker. While the Biden administration has pledged to donate doses abroad, boosters take up airtime in expert discussions and in the media—including in this publication. Boosters distract from discussions of what it will take to vaccinate the world, which is our true way out of the pandemic.
More vaccine reporting
-
Moderna for the middle children
Good news for kids hoping for jabs in arms (which used to sound like an oxymoron before this spring): Moderna has announced promising results for its trial in adolescent-aged children. In around 4,000 adolescents, the vaccine proved to be 94.1% effective in preventing disease. No cases in the vaccinated group were found two weeks after the second shot, while 4 cases were found in the unvaccinated control group.
On Tuesday, May 25, Moderna showed in a clinical trial that its mRNA vaccine is safe and effective in people ages 12 to 17. The company will apply for FDA emergency use authorization in June. This follows the semi-recent authorization of the Pfizer-Biontech vaccine for the same age group, which happened at the end of March.
While children tend to have less severe complications from COVID-19 on the whole, serious illness is still quite possible. And even though rates across the country are falling due to more widespread vaccination, it’s still important that kids get vaccinated as herd immunity is not quite in our grasp yet.
The availability of another vaccine may help more people in this age group get protected; however, the rest of the world has nowhere near the access to vaccines that U.S. citizens over age 12 do right now. In April, health policy experts estimated that the United States might have an excess of up to 300,000 extra vaccines.
That being said, adolescents should still get vaccinated if it is available to them. This problem isn’t the fault of citizens wanting to get protection; it’s about the failures of governments and systems to provide vaccine equity.
More vaccine reporting
- Sources and updates, November 12Sources and updates for the week of November 12 include new vaccination data, a rapid test receiving FDA approval, treatment guidelines, and more.
- How is the CDC tracking the latest round of COVID-19 vaccines?Following the end of the federal public health emergency in May, the CDC has lost its authority to collect vaccination data from all state and local health agencies that keep immunization records. As a result, the CDC is no longer providing comprehensive vaccination numbers on its COVID-19 dashboards. But we still have some information about this year’s vaccination campaign, thanks to continued CDC efforts as well as reporting by other health agencies and research organizations.
- Sources and updates, October 8Sources and updates for the week of October 8 include new papers about booster shot uptake, at-home tests, and Long COVID symptoms.
- COVID source shout-out: Novavax’s booster is now availableThis week, the FDA authorized Novavax’s updated COVID-19 vaccine. Here’s why some people are excited to get Novavax’s vaccine this fall, as opposed to Pfizer’s or Moderna’s.
- COVID-19 vaccine issues: Stories from COVID-19 Data Dispatch readers across the U.S.Last week, I asked you, COVID-19 Data Dispatch readers, to send me your stories of challenges you experienced when trying to get this fall’s COVID-19 vaccines. I received 35 responses from readers across the country, demonstrating issues with insurance coverage, pharmacy logistics, and more.
- Sources and updates, November 12