- Household Pulse Survey updates, expands Long COVID data: This week, the CDC and Census released an update of their Household Pulse Survey results on how Long COVID is impacting Americans. In addition to more recent data on Long COVID prevalence, the update includes new information on how adults with the condition find it limiting their day-to-day activities. The data shows that, out of all adults currently experiencing Long COVID symptoms, over 80% have some activity limitations and 25% have “significant” activity limitations. (For more context on this dataset, see my post from June.)
- NIH shares update on RECOVER study: Speaking of Long COVID, the National Institutes of Health’s Directors Blog shared a post this week with updates on its flagship RECOVER study to learn more about the condition. Major updates include: RECOVER’s current recruitment goal is 17,000 adults and 18,000 children; the NIH recently awarded more than 40 grants to research projects examining the condition’s underlying biology; and RECOVER is utilizing electronic health records to track patients over time. While this is all valuable progress, patient advocates have expressed concerns about limited involvement by post-viral chronic illness experts in RECOVER so far.
- Paxlovid is going under-utilized, study finds: A new report from the health records company Epic Research provides evidence that Paxlovid reduces severe COVID-19 outcomes: patients over age 50 who received the antiviral drug were about three times less likely to be hospitalized, compared with those who didn’t. The study also found, however, that eligible Americans aren’t taking advantage of this treatment. Out of about 570,000 people who “could have received Paxlovid” between March and August 2022, only 146,000 (about one in four) actually got prescriptions. Paxlovid needs to be better advertised and easier to access.
- New COVID-19 pill added to Medicines Patent Pool: And a new COVID-19 treatment option is becoming available internationally. Shionogi, a Japanese pharmaceutical company, recently signed an agreement with the Medicines Patent Pool, an international public health organization that facilitates increased drug access in low- and middle-income countries. The agreement allows other drug companies to make Shoinogi’s antiviral COVID-19 pill, called ensitrelvir fumaric acid, which has seen some promising results in clinical trials so far. Paxlovid and Molnupiravir (Merck’s antiviral pill) are already licensed by the pool.
- Patient access to electronic health records expands: This past Thursday, new federal rules took effect requiring healthcare companies to “give patients unfettered access to their full health records in digital format,” as STAT News reporter Casey Ross put it. This is a major milestone for the democratization of health data, as patient records have historically been locked in a labyrinth of private databases—though more public education is needed to help people actually take advantage of the new rules. Personally, I hope this is a first step towards more record-sharing between health institutions, which could be a key step for more comprehensive analysis in the future.
Tag: electronic health records
-
Sources and updates, October 9
-
New Long COVID studies demonstrate danger of breakthrough cases
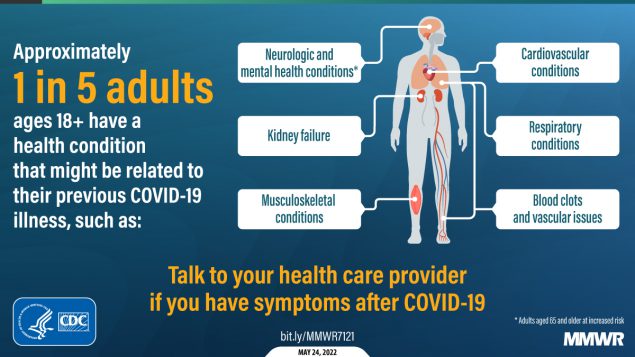
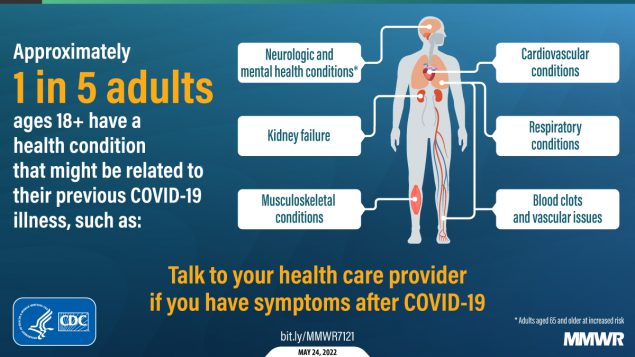
About one in five adults who have COVID-19 will face a health condition potentially related to long-term symptoms, a new CDC study found. Two new studies on Long COVID, published this week, provide an important reminder of the continued dangers this condition poses to people infected with the coronavirus—even after vaccination. Neither study provides wholly new information, but both are more comprehensive than many other U.S. papers on this condition as they’re based on large databases of electronic health records.
First: a team at the Veterans Affairs (VA) Health Care System in St. Louis, Missouri used the VA’s extensive health records database to study breakthrough COVID-19 cases. The VA database includes more than 1,400 healthcare facilities serving veterans across the country; this St. Louis team has previously used it to characterize Long COVID symptoms more broadly, to study long-term heart disease risks of COVID-19, and for other research.
In the new paper, published this week in Nature Medicine, the researchers put together a cohort of about 34,000 people who had breakthrough COVID-19 infections. They compared this group to larger control groups of people who hadn’t been infected and people who had been infected prior to vaccination, along with comparisons to the seasonal flu.
Vaccination does reduce the risk of Long COVID, the researchers found: people with breakthrough cases were 15% less likely to report Long COVID symptoms than those who were infected prior to vaccination. Breakthrough Long COVID patients were notably less likely to have blood clots and respiratory symptoms than non-breakthrough patients.
But a risk reduction of 15% is pretty minimal, compared to the protection that vaccination offers against COVID-related hospitalization and death. Moreover, for most Long COVID symptoms, patients who had breakthrough infections showed relatively little difference to those who had non-breakthroughs, the researchers found.
“Overall, the burden of death and disease experienced by people with breakthrough SARS-CoV-2 infection is not trivial,” lead researcher Dr. Ziyad Al-Aly wrote in a Twitter thread summarizing the study. That’s scientist speak for, “A breakthrough COVID-19 case can really fuck you up in the long term!” Later in his thread, Dr. Al-Aly advocated for additional public health measures—beyond simply vaccines—to reduce Long COVID risks.
And second: a paper from the CDC’s COVID-19 Emergency Response Team, published in the CDC’s Morbidity and Mortality Weekly Report (MMWR) last week, used electronic health records to examine overall Long COVID risk after an infection. These health records came from Cerner Real-World Data, a dataset including about 63.4 million records from over 100 health providers.
The CDC researchers identified about 353,000 adults who had received either a COVID-19 diagnosis or a positive test result between March 2020 and November 2021. They matched this group of COVID-19 patients with a larger cohort of people who hadn’t tested positive, then looked at the COVID-19 patients’ risks of developing further symptoms more than a month after they were diagnosed.
The findings are striking: About one in five COVID-19 survivors between the ages of 18 and 64 developed at least one “incident condition” (or, prolonged symptoms) that could be connected to their coronavirus infection. For COVID-19 survivors over age 65, that risk is one in four.
Among the patients who potentially developed Long COVID, common symptoms were blockages in the lungs and other respiratory issues. Seniors were also likely to develop neurological and mental health symptoms, and the CDC researchers warned that Long COVID in this older age group could be linked to an increased risk of strokes and neurocognitive conditions, such as Alzheimers.
In their paper, the CDC authors noted that patients represented in this health records database may not represent the U.S. overall, and that the methods used to identify possible Long COVID symptoms might be “biased toward a population that is seeking care.” Similar caveats apply to the VA study.
Still, both studies clearly show the risk of just “letting COVID-19 rip” through the U.S. population, even after widespread vaccination. Studies like these should be headlines in every news publication, warning people that COVID-19 is not as mild as many of our leaders would like us to believe.
Also, for journalists covering the pandemic: I highly recommend listening to this interview with Long COVID journalist and advocate Fiona Lowenstein, which aired on the WNYC show On the Media this weekend. (And I’m not just saying that because they plugged my recent story on the RECOVER study!) The Long COVID source list that Fiona and I collaborated on also continues to be a great resource for reporters covering this topic.
More Long COVID reporting
-
New study demonstrates potential for measuring breakthrough risk
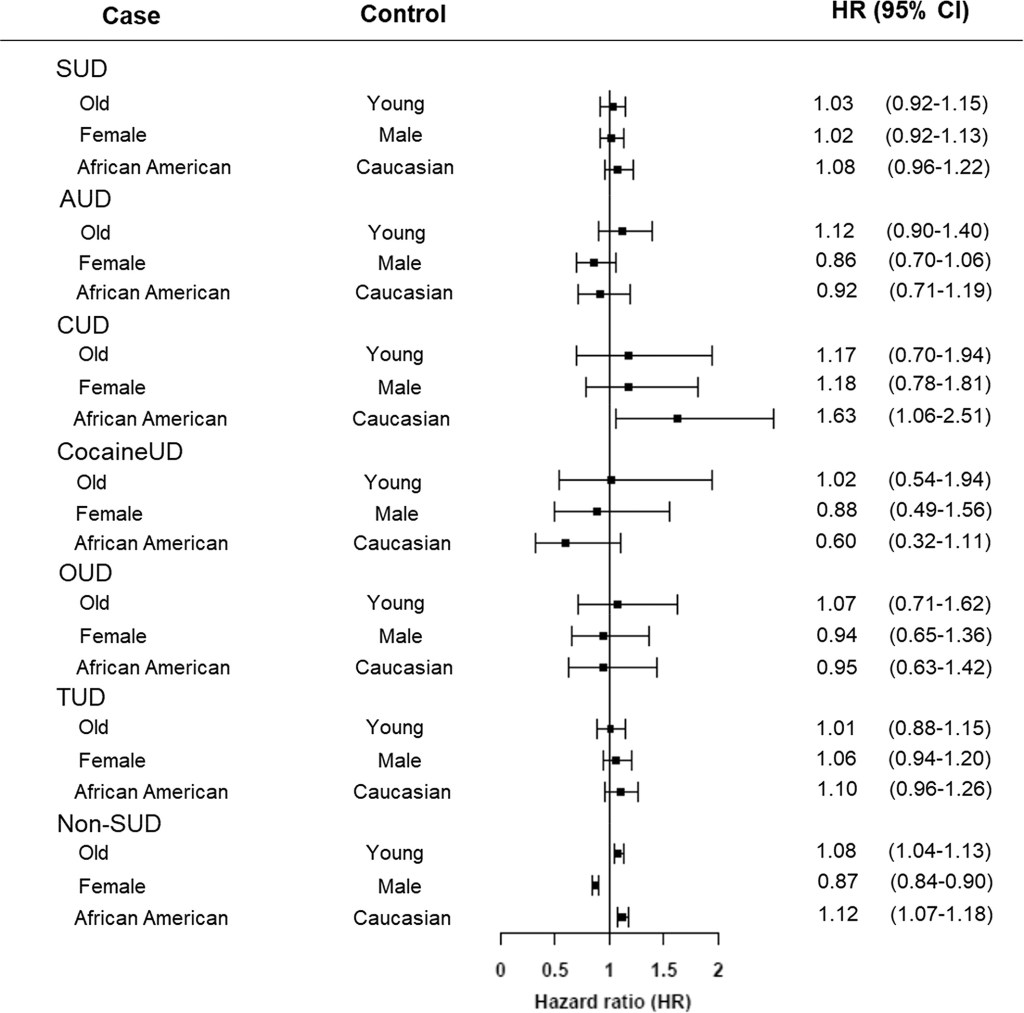
Adults with substance use disorders have an increased risk of breakthrough cases, according to a new study published this week in the journal World Psychiatry. Though the chances of a COVID-19 case after vaccination were very low in this group, these patients’ odds of a breakthrough case were about twice as high as the odds for adults without substance use disorders, researchers from the National Institutes of Health (NIH) found.
This study is the first I’ve seen to delineate breakthrough case risk in a specific, vulnerable population—besides studies demonstrating higher risk for older adults. As I wrote two weeks ago, a lack of specific data on breakthrough cases has contributed to confusion and debate surrounding who should be eligible for a booster shot in the U.S.
So, how did these NIH researchers determine the risk for people with substance abuse? They used anonymous, electronic health records from 63 healthcare organizations across the U.S., compiled in the TriNetX Analytics platform. The study included health records from about 30,000 patients with substance use disorders, compared with 550,000 patients without these disorders. From this large pool of anonymous data, the researchers were able to determine breakthrough case risk among different patient demographics, different substance use disorders, and more.
I got a chance to talk to Dr. Nora Volkow, director of the NIH’s National Institute on Drug Abuse and one of the study’s lead authors, about this methodology, as I covered the paper for DailyMail.com. I asked her if she expected to see similar studies examining breakthrough case risk for other health risks and occupations.
“Absolutely,” Dr. Volkow said. She told me she’s already seen other papers comparing the risk of a breakthrough with Delta compared to other variants, and that more research looking at specific patient groups may be ongoing. Still, using electronic health records has its drawbacks.
“We are basically basing [the analysis] on the electronic health records,” she said. “But it could be useful to complement this with studies that actually are genotyping, getting information about, what was the virus that is responsible?” In other words: health records from hospitals and clinics typically are not matched with genetic sequencing information, making it difficult to link specific variants with breakthrough case risk.
As for why patients struggling with substance abuse have a higher risk of breakthrough COVID-19: Dr. Volkow said this is largely due to socioeconomic factors, such as lack of access to healthcare, low income, and homelessness. Drugs and alcohol are also capable of weakening patients’ immune systems, though; marijuana in particular can hinder immune system regulation.
More vaccination data