- Healthcare worker burnout trend backed up by new data: The COVID-19 pandemic has exacerbated a growing burnout crisis among healthcare workers in the U.S., as many articles and scientific papers have explored in the last couple of years. Two studies from the past week add more data to back up the trend. CDC researchers shared the results of a survey of about 2,000 workers, finding that workers were more likely to report poor mental health and burnout in 2022 than in 2018, while harassment and a lack of support at work contributed to increased burnout. Another research group (at the University of North Carolina at Greensboro and Washington University in St. Louis) also surveyed healthcare workers and found that many experienced food insecurity and financial challenges; workers with worse employer benefits were more likely to increase these challenges.
- Viral load not necessarily associated with symptoms: This paper is a rare, relatively recent update on how COVID-19 symptoms connect to viral load, or the amount of virus that a patient has in their respiratory tract. The higher a patient’s viral load, the more likely they are to infect others, making this an important metric for contagiousness. Researchers at Emory University studied viral loads in about 350 people diagnosed with Omicron variants between April 2022 and April 2023. Patients tended to have their highest viral loads around the fourth day of symptoms, a change from studies done on earlier variants (when viral loads tended to peak along with symptoms starting). As Mara Aspinall and Liz Ruark explain in their testing newsletter, these results have implications for rapid at-home tests, which are most accurate when viral loads are high: if you’re symptomatic but negative on a rapid test, keep testing for several days, and consider isolating anyway.
- Updated vaccines are key for protection: Another recent paper, in The Lancet Respiratory Medicine, examines how last year’s bivalent COVID-19 vaccines worked against recent coronavirus variants using data from the Kaiser Permanente health system. The study included records from about 123,000 people who had received at least the original vaccine series, examining health system visits from August 2022 to April 2023. People who received an updated vaccine in fall 2022 were significantly less likely to have severe COVID-19, the researchers found. “By mid-April, 2023, individuals previously vaccinated only with wild-type vaccines had little protection against COVID-19,” the researchers wrote. This year’s updated vaccine may have a similar impact through spring 2024.
- Gut fungi as a potential driver for Long COVID: Long COVID, like ME/CFS and other chronic conditions, may be associated with problems in patients’ gut microbiomes, i.e. the communities of microorganisms that live in our digestive systems. A new paper in Nature Immunology from researchers at Weill Cornell Medicine hones in on one fungal species that may be particularly good at causing problems. The species, Candida albicans, can grow in the intestines of severe COVID-19 and Long COVID patients, triggering to inflammation and other immune system issues. This paper describes results from patient samples as well as a mouse model mimicking how this fungal species grows in COVID-19 patients’ guts.
- Another potential Long COVID biomarker: One more notable Long COVID paper from this week: researchers at the University of Alberta studied blood samples from people with the condition, and compared their results to people who had acute COVID-19 but didn’t go on to develop long-term symptoms. The scientists used machine learning to develop a computer model differentiating between blood composition of people who did and didn’t develop Long COVID. They identified taurine as one specific amino acid that might be particularly important, as levels of taurine were lower among patients with more Long COVID symptoms. The study could be used to inform diagnostic tests of Long COVID, as well as potential treatments to restore taurine.
Tag: booster shots
-
Sources and updates, October 29
-
Sources and updates, October 8
- Vaccination disparities in long-term care facilities: A new study in the CDC’s Morbidity and Mortality Weekly Report shares vaccination patterns from about 1,800 nursing homes, assisted living facilities, and other long-term care facilities across the U.S., focusing on the bivalent booster (or, last fall’s vaccine). The CDC researchers found significant disparities in these vaccinations: vaccine coverage was lowest among Black and Hispanic residents compared to other demographics, and was lowest in the South and Southeast compared to other regions. Future vaccination campaigns need to make it easy for these groups to get their shots, the authors suggest; but based on how the 2023 rollout has gone so far, this trend seems likely to continue.
- Reasons for poor bivalent booster uptake: Speaking of last fall’s boosters, a study from researchers at the University of Arizona suggests reasons why people didn’t get the shots last year. Researchers surveyed about 2,200 Arizona residents who had received at least one COVID-19 vaccine dose. Among the survey respondents who didn’t get last year’s booster, the most common reason for not doing so was a belief that a prior infection made the shot unnecessary (40%), concerns about vaccine side effects (32%), believing the booster wouldn’t provide additional protection over prior shots (29%), and safety concerns (23%). As with the study above, this paper shows weaknesses in the U.S.’s recent vaccine promotion strategies.
- At-home tests are useful but far from perfect: Researchers at Nagoya University and the University of Oxford used mathematical models to study how different safety measures impact chances of COVID-19 outbreaks. The researchers developed models based on contact tracing data reflecting how Omicron spreads through groups. Rapid, at-home, antigen tests are a useful but imperfect method for reducing outbreak risk, the study found, with daily testing reducing the risk of a school or workplace outbreak by 45% compared to a scenario in which new cases are identified by symptoms only. “In high-contact settings, or when a new variant emerges, mitigations other than antigen tests will be necessary,” one of the scientists said in a statement.
- Long-term symptoms from non-COVID infections: The prevalence of Long COVID has led many scientists to develop new interest in chronic conditions that may arise after other common infections, such as the flu and other respiratory viruses. One recent study from Queen Mary University of London identifies a potential pattern, using data from COVIDENCE UK, a long-term study tracking about 20,000 people through monthly surveys. Researchers compared symptoms between people who had a COVID-19 diagnosis and those with other respiratory infections, looking at the month following infection. They found similar risks of health issues in the one-month timeframe for both groups, though specific symptoms (loss of taste and smell, dizziness) were more specific to Long COVID. Of course, some people in the “non-COVID” group could have had COVID-19 without a positive test; still, the data indicate more, longer-term research is needed.
- Autoimmune disorders following COVID-19: In another Long COVID-related paper, researchers at Yonsei University and St. Vincent’s Hospital in South Korea found that patients had increased risks of autoimmune and autoinflammatory disorders following COVID-19 cases. The study used patient records from South Korea’s national public health system, comparing about 354,000 people who had COVID-19 diagnoses to 6.1 million controls. COVID-19 patients had a significant risk of new autoimmune issues within several months after infection; new diagnoses included alopecia (or hair loss), Crohn’s disease (inflammatory bowel issues), sarcoidosis (overactive immune system), and more. These conditions should be considered by doctors evaluating potential Long COVID patients, the researchers wrote in their paper.
- New climate vulnerability index: This last item isn’t directly COVID-19 related, but may be useful in evaluating community risks for public health threats. The Environmental Defense Fund, Texas A&M University, and other partners have launched the U.S. Climate Vulnerability Index, a database providing Census tract-level information about how our changing climate will impact different communities. Communities are ranked from low to high climate vulnerability, with detailed data available on sociodemographic characteristics as well as potential extreme weather events and health trends.
-
COVID source shout-out: Novavax’s booster is now available
This week, the FDA authorized Novavax’s updated COVID-19 vaccine. The CDC’s fall vaccine recommendations were already set up to include Novavax once it was authorized, so pharmacies and health providers can start administering it without any additional hurdles at the federal level.
Novavax’s new vaccine, like the options from Pfizer and Moderna for this fall, is designed to protect against XBB.1.5, a recently circulating variant that is closely related to most of the strains causing disease in the U.S. right now. But unlike the Pfizer and Moderna vaccines (which use mRNA technology), Novavax’s uses a piece of viral spike protein to teach recipients’ immune systems how to recognize the coronavirus.
Some scientists and health advocates I follow have been particularly looking forward to the Novavax authorization, hoping to get their shot rather than one of the mRNA options. There are two main reasons for this choice, based on my reading:
- The Novavax vaccine may have fewer or easier side effects than the mRNA vaccines. This is particularly appealing for some people who had poor reactions to earlier mRNA vaccine doses (including, in some cases, long-term issues similar to Long COVID), and some people with chronic conditions.
- Some experts say that “mixing and matching” different types of vaccines might lead to a more robust, long-term immune response against the coronavirus, compared to sticking with one vaccine type.
A recent article in Science goes into more detail about these considerations. Writer Jennifer Couzin-Frankel walks through scientific studies that look at Novavax compared to the other vaccine options, and explains some of the questions that we don’t have sufficient data to answer yet. For example, as fewer people have received Novavax vaccines compared to the mRNA options, it’s harder to see signals for potential rare adverse reactions. More studies are coming in that will help address these questions, but for now, many people are making personal choices about which vaccine to get this fall.
-
COVID-19 vaccine issues: Stories from COVID-19 Data Dispatch readers across the U.S.

Last year, just 17% of the U.S. population received a bivalent booster. Will this year’s uptake be better? Last week, I asked you, COVID-19 Data Dispatch readers, to send me your stories of challenges you experienced when trying to get this fall’s COVID-19 vaccines. I received 35 responses from readers across the country, demonstrating issues with insurance coverage, pharmacy logistics, and more.
I’ve published the full responses in the table below. Here are a few common themes that I saw in these stories:
- Pharmacies aren’t receiving enough vaccines. Several readers shared that their pharmacies had inadequate vaccine supply to accommodate all the people who made vaccination appointments, or who wanted appointments. Vaccine supply may also be unpredictable—a pharmacy may think they’re getting more shots, but in fact not receive them—leading to appointment cancellations.
- Insurance providers weren’t prepared for this vaccine rollout. Despite months of advance notice that a fall COVID-19 vaccine was coming, many insurance companies apparently failed to prepare billing codes or other system updates that would allow them to cover the shots. A couple of people who shared insurance issue stories are on Medicare—representing a population (i.e. seniors) who should be at the front of the vaccine line.
- Very limited, confusing vaccine availability for young kids. Several readers shared that they were able to get vaccinated, but their children under 12 have not received a vaccine yet. While the FDA and CDC have authorized this fall’s COVID-19 vaccines for all Americans ages six months and older, younger children require a different vaccine formulation from adults. And this formulation appears either entirely unavailable or very difficult to access, depending on where you live.
- People living in less dense areas may need to travel. A few readers shared that, as they searched for vaccine appointments in their areas, the closest pharmacies with doses available were miles away—over 10 miles, in one case. This is a significant barrier for people fitting vaccine appointments into their work schedules.
- Information may be inconsistent. Vaccine availability listed in one place (such as a pharmacy chain’s website or the federal vaccines.gov website) may be inaccurate in another. Some readers shared that they spent extra time on the phone with pharmacies or health providers to get accurate information—another barrier.
- Pharmacies don’t have enough staff for this. Even readers who were able to receive COVID-19 vaccines often had to wait a long time at their pharmacies. Several shared that their pharmacies appeared to be understaffed, dealing with the COVID-19 shots along with routine prescriptions and other duties. The days of mass vaccination sites, efficiently run by public health departments, are long over.
- Kaiser Permanente members face delays. One company that appears to be causing outsized problems is Kaiser Permanente, one of the biggest insurers and health providers on the West Coast. Several readers shared that Kaiser was not providing new COVID-19 vaccines until early October, and would not cover the shots if their members went to another location. That’s a big delay, and it may be further impacted by a coming strike at the company.
- These vaccines are expensive. If you decide to pay for a COVID-19 shot out-of-pocket (as some readers did), it costs almost $200. Even the federal government is paying about triple the cost of last year’s COVID-19 vaccines per shot, for the doses it is covering, STAT News reports. The U.S. may have received a “bad deal” here, STAT suggests, considering all of the federal funding that’s supported vaccine research and development.
As I wrote last week, some news outlets have covered these challenges, but this issue really deserves more attention. The updated COVID-19 vaccines are basically the U.S. government’s only strategy to curb a surge this winter, and they should be easily, universally accessible. Instead, many people eager to get vaccinated are going through multiple rounds of appointments, phone calls, pharmacy lines, and more.
For every one of these readers who has persisted in getting their shot, there are likely many other people who tried once and then gave up. And those people who don’t receive the vaccine will be at higher risk of severe illness, death, and long-term symptoms from COVID-19 this fall and winter. This is a public health failure, plain and simple.
And it’s important to emphasize that this failure is not surprising. Many health commentators predicted that these challenges would arise as the federal public health emergency ended and COVID-19 tools transitioned from government-funded to covered-by-insurance. For more context on why this is happening, I recommend the Death Panel podcast’s latest episode, “Scenes from the Class Struggle at CVS.”
If you’re a reporter who would like to connect with one of the COVID-19 Data Dispatch readers who shared a story, please email me at betsy@coviddatadispatch.com. Most of the people in the database below shared an email or other contact info.
[table id=10 responsive=collapse responsive_breakpoint=all /] -
New COVID-19 vaccines are now available: 10 key facts and statistics about these shots

Data from a CDC presentation suggest that people of all ages, including children, receive a benefit from updated COVID-19 vaccines. We now have two new COVID-19 vaccines available for this year’s respiratory virus season, one from Pfizer and one from Moderna, which are expected to perform well against current variants. The FDA approved both vaccines this week, and the CDC recommended them for almost all Americans. A third option, from Novavax, may become available in the coming weeks as well.
The federal government aims to present this fall’s shots as the next iteration in routine, annual COVID-19 vaccines—similar to the routine we’re all used to for flu shots. In fact, I’ve seen some news suggesting that the federal health agencies don’t want us to call these shots “boosters,” instead calling them “updated” shots or annual shots.
But this fall’s vaccine rollout is likely to be anything but routine, as it’s the first rollout following the end of the federal COVID-19 public health emergencies. The government is no longer purchasing shots and distributing them for free; now, insurance companies will have to cover the shots.
As a result, many Americans—especially those without health insurance—will have a harder time accessing these vaccines than they have for previous shots. Plus, the federal emergency’s end will make it harder for us to track how the vaccines are performing, as the coronavirus continues to evolve into new variants.
With all of these complications in mind, here are ten key facts and statistics that you should know about this fall’s COVID-19 vaccines.
Pfizer and Moderna’s shots have been approved and recommended for all Americans, ages six months and older.
Despite some debates among scientists about whether younger people really need updated COVID-19 shots, the FDA has approved these vaccines—and the CDC has recommended them— for all age groups. This is important because CDC recommendations are often the basis for insurance coverage, as experts explained at a webinar hosted by the National Press Foundation on Tuesday.
The shots exclusively target XBB.1.5, a coronavirus lineage that is common in the U.S. and globally right now.
According to the CDC’s genomic surveillance program, almost all cases in the U.S. in recent weeks have been caused by XBB.1.5 or related variants from the XBB lineage. Variants like EG.5 and FL.1.5.1 are also XBB descendants, which have been given nicknames to make it a bit easier for scientists to keep track of them.
It’s also important to note that, unlike last year’s boosters, this fall’s shots are monovalent vaccines—meaning they only target XBB.1.5. The shots no longer target the original strain of SARS-CoV-2 that first circulated in 2020. Scientists generally approve of this choice, as the virus has mutated so much since that time.
Moderna’s booster led to a 17-fold increase in antibodies against XBB.1.5 and XBB.1.6.
The vaccine companies presented data to the CDC’s vaccine advisory committee on Tuesday. Moderna’s presentation included results from a study testing its new vaccine against several different variants, using blood samples from people who received the booster.
About one month after vaccination with Moderna’s booster, the participants had about 17.5 times more neutralizing antibodies against XBB.1.5, 16.7 times more against XBB.1.6, 14 times more against EG.5.1, and 10 times more against BA.2.86. Pfizer also presented data, suggesting that their vaccine should similarly perform well against current variants.
The new vaccines should lead to similar side effects as we’re used to from past mRNA shots.
Based on data that the vaccine companies presented to the CDC’s committee, this fall’s Pfizer and Moderna vaccines should lead to similar side effects—headache, fatigue, muscle pain, etc.—as many of us have expected from past rounds of COVID-19 shots. The companies, along with the CDC and FDA, will continue to monitor these vaccines for any safety issues that may emerge as people start to get them.
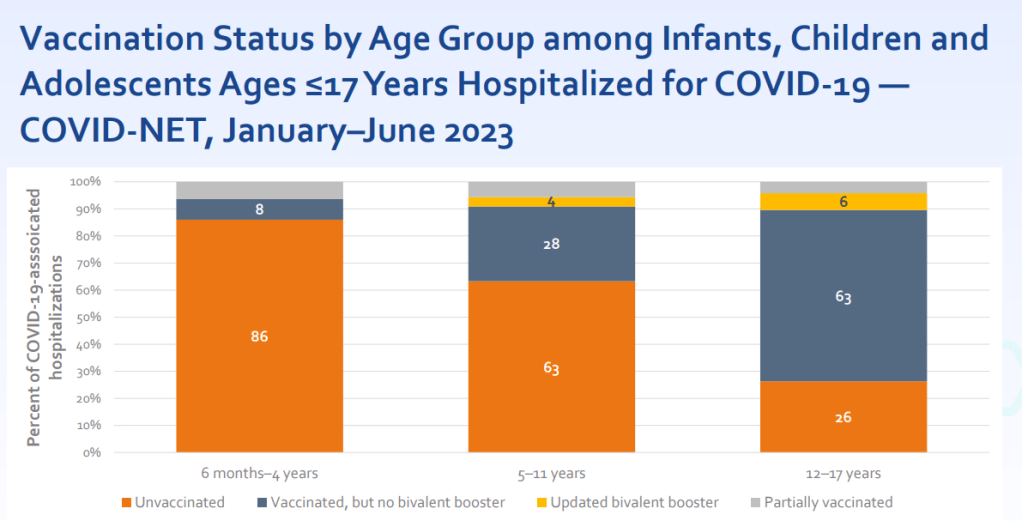
Young, unvaccinated children are at higher risk for COVID-19.
One of the CDC presentations focused on how this fall’s vaccines may benefit young children. Last fall and winter, hospitalization rates were higher for COVID-19 than for the seasonal flu across all young age groups, from infants (under six months) to 12-17 years old. The vast majority of the children hospialized were not vaccinated or hadn’t received last year’s booster.
For some CDC advisory committee members, these data were convincing in suggesting that this fall’s vaccine should be recommended for children, experts told STAT News. Vaccines updated to match current variants have a clear benefit for all age groups.
Long COVID remains a significant risk for Americans across age groups.
Another CDC presentation discussed Long COVID, as one of the potential adverse outcomes of a COVID-19 case. The CDC shared new data from a national survey conducted in 2022, which suggests that 9% of Americans ages 35 to 49 have experienced Long COVID symptoms (defined as symptoms lasting at least three months after a COVID-19 case). Adults ages 50-64 and 18-34 also reported high levels of Long COVID, at 7.4% and 6.8% ever experiencing symptoms, respectively.
Many studies have shown that vaccination lowers risk of Long COVID, though it does not by any means eliminate this risk. While it’s good to see the CDC incorporating Long COVID into its vaccine risk/benefit discussions, much more research is needed to better understand how to prevent this debilitating condition.
A Novavax vaccine is still in the pipeline.
Novavax also presented data to the CDC’s advisors this week, suggesting that its vaccine (also based on XBB.1.5) should perform similarly to the Pfizer and Moderna options. But unlike the Pfizer and Moderna vaccines, Novavax’s has yet to receive FDA approval. The company has said it’s still planning to distribute its vaccine this fall, but it’s unclear when the FDA may authorize it.
Some people are eager to receive the Novavax vaccine this fall, rather than Pfizer or Moderna’s, because this vaccine uses a different mechanism to boost the immune system. It may also lead to fewer side effects than the mRNA vaccine, making it a potentially good option for people who’ve had particularly strong reactions. (I know a couple of readers have sent me questions about this, and aim to do a deep-dive on Novavax in a future issue.)
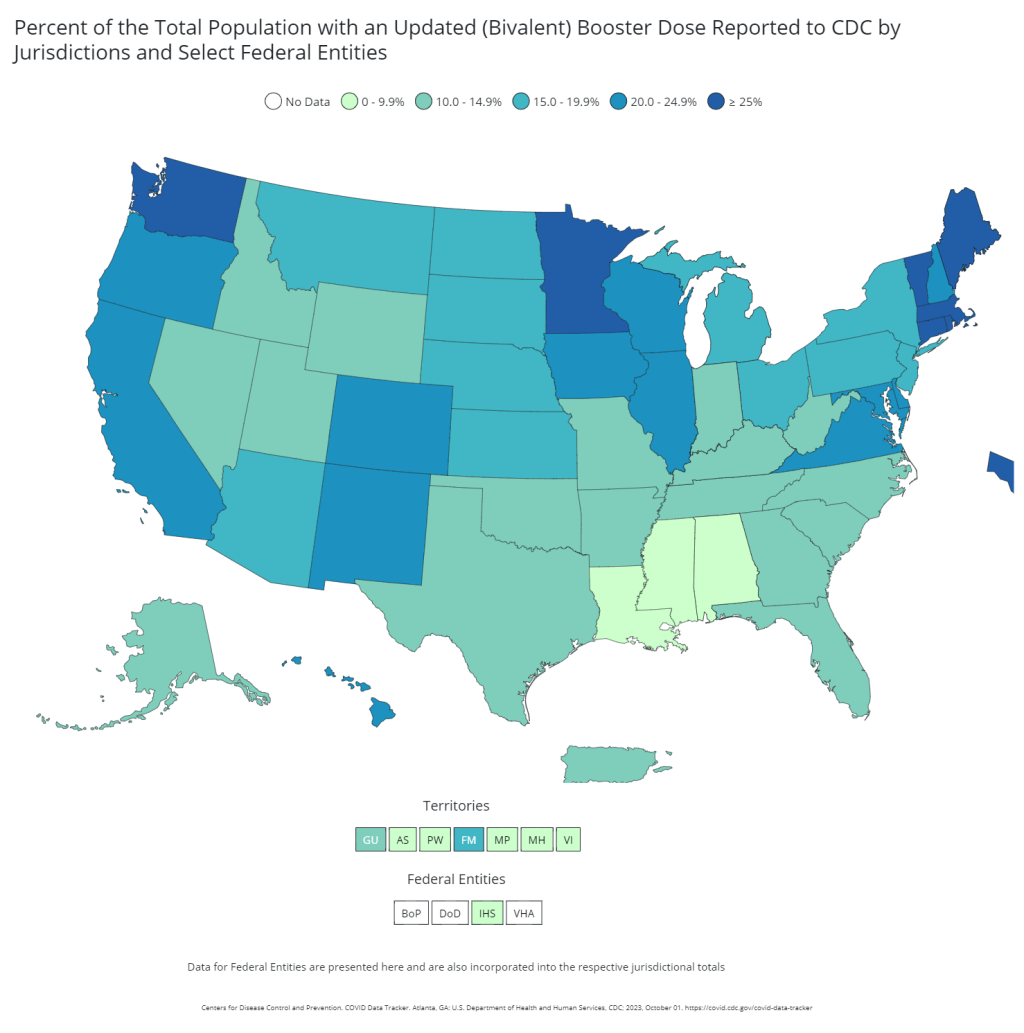
Only 17% of Americans received last fall’s bivalent booster.
The booster uptake last year was low, according to the CDC. Even among seniors, only 43% received the booster. Can we do better this year?
A POLITICO/Morning Consult poll found that about 60% of respondents said they “probably or definitely” would get this year’s vaccine. (The poll included about 2,000 registered voters from across the U.S.) But it’s likely that access issues could get in the way for many people, as getting this COVID-19 vaccine will be much more challenging than it’s been in past rollouts.
HHS program should provide free vaccines for 25-30 million adults.
The Department of Health and Human Services has officially launched its “Bridge to Access” program, designed to provide free COVID-19 shots to uninsured Americans. Through this program, the HHS is essentially buying a small number of shots and distributing them to pharmacies, federally supported health centers, and other providers. You should be able to view these providers at vaccines.gov, according to the HHS. But I’ll be curious to see how well that actually works.
This year’s vaccine rollout will be much harder to track.
In the past, I’ve written about how the U.S. has failed to monitor breakthrough cases, or COVID-19 infections that occur after someone is vaccinated (and the hospitalizations, deaths, and long-term symptoms that may result). This year, not only are we failing to track breakthrough cases—the U.S. no longer has any national case data at all. We also no longer have vaccination data, as the CDC is not collecting this information from state and local health systems.
So, how will we know how this year’s vaccine rollout goes? It’ll likely be a lot of guesswork, extrapolating from a few state/local health departments, polling data, and other smaller-scale research to estimate how many people are getting vaccinated nationally. This challenge is just another example of the damage that the federal government has done in the last year by dismantling many of its COVID-19 data systems.
-
New data on BA.2.86 suggest the fall booster may work well
Since BA.2.86 emerged a couple of weeks ago, scientists around the world have been racing to evaluate this variant. Several teams posted data in the last week, and the news is promising: while BA.2.86 does have an advantage over past variants, the lab findings suggest that vaccines (including the upcoming boosters) and past infections provide protection against it.
The new studies come from research groups in the U.S., China, Japan, Switzerland, and South Africa. These scientists studied BA.2.86 by growing the variant in petri dishes and evaluating it against antibodies from blood samples. Overall, they found that BA.2.86 can infect people who were recently infected with XBB.1.5 and its relatives, but this variant isn’t as successful at getting into human cells as XBB.1.5.
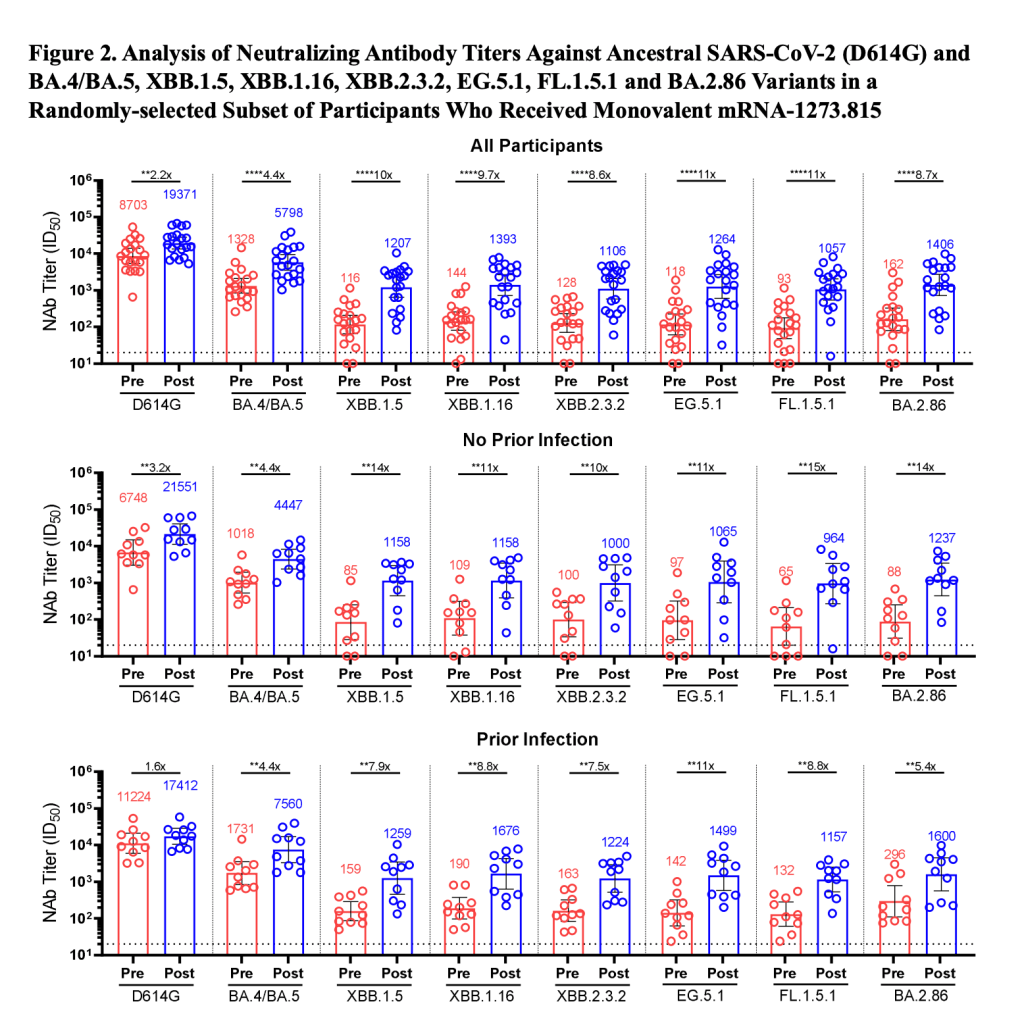
Another notable study came from researchers at Moderna, who evaluated how the company’s upcoming booster shot performs against BA.2.86. This team found that the booster—which is designed from XBB.1.5—helps the immune system prepare for XBB variants as well as BA.2.86. While lab studies like this one don’t translate perfectly to real-world effectiveness, the data do suggest that Moderna’s booster should protect well against BA.2.86 infection for a few weeks after vaccination, and against severe disease for longer.
You might have seen the figure below shared around on social media in the last few days. This chart, from the Moderna team, shows how the new booster improves immunity toward several variants. For example, patients who received the booster had 8.7 times more neutralizing antibodies against BA.2.86 and 10 times more neutralizing antibodies against XBB.1.5 than those who had not received it.

This figure, from a preprint by Moderna scientists, shows how the company’s upcoming fall booster performs against different variants. Pfizer has also tested their new booster against BA.2.86 and found similar results, according to a report from Reuters. This company’s results have yet to be shared in a scientific paper, though.
The studies I’ve discussed here are all preprints, meaning the results have yet to be peer-reviewed (outside of the informal review process that happens on social media for this type of urgent research). It’s also worth noting that lab studies look at immune system signals, rather than actually tracking who’s getting this new variant and their disease outcomes.
Even if BA.2.86 is not “the next Omicron,” as some scientists suggested based on its mutations, it could still contribute to a new uptick in cases this fall. And all cases carry the risk of severe illness, Long COVID, and other poor outcomes. The new boosters are likely to help reduce risk (which is good news), but other measures are still needed.
References about the new studies:
- The BA.2.86 variant and the new booster (Ground Truths by Eric Topol)
- BA.2.86 update (Your Local Epidemiologist by Katelyn Jetelina)
- More COVID-19 studies suggest BA.2.86 may be less immune-evasive than feared (University of Minnesota CIDRAP)
- Covid is on the rise again, but it’s different now (Vox)
-
Answering reader questions: Incubation period, vaccines coming this fall, nasal sprays
I received a couple of reader questions in recent weeks that I’d like to answer here, in the hopes that my responses will be more broadly helpful. As a reminder, if you ever have a COVID-19 question that you’d like to ask, you can email me at betsy@coviddatadispatch.com, or send it anonymously through this Google form.
COVID-19’s incubation period
One reader asked:
I’d love to learn more about COVID’s incubation period. I have read that it’s 2 to 14 days … but the median time seems to be on the low end (and could be as low as 24 hours?) How likely is it that it’s more like 14 days? I’d love to better understand this so that I know how to better handle exposures… Should I avoid someone who has had an exposure for two full weeks?
This is a tricky question for two reasons. First, the incubation period—or the time between exposure to COVID-19 and starting to show symptoms of infection—does indeed vary a lot. One review of studies on this topic, posted as a preprint in May, found a range from two to seven days, though it can be even longer. The CDC recommends precautions for up to ten days after exposure.
Second, the incubation period has changed as the coronavirus has mutated. The virus is constantly evolving to keep infecting us even as people build up immunity; shortening the incubation period is one of its strategies. Omicron has a notably shorter period than past variants; Katherine Wu at The Atlantic wrote an article about this in December 2021 that I think is still informative.
The preprint I cited above found that Omicron had an average incubation period of 3.6 days, shorter than other variants. I think it’s reasonable to assume that this period has continued to get shorter as Omicron has evolved into the many lineages we’re dealing with now. But the pace of research on this topic has slowed somewhat (with less contact-tracing data available for scientists to work with), so it’s hard to say for certain.
So, with these complexities in mind, how should one handle exposures? My personal strategy for this (noting that I’m not a doctor or qualified to give medical advice, just sharing my own experience) is to rely on a combination of timing, testing, and symptom monitoring. For the first couple of days after exposure, you wouldn’t be likely to have a positive test result even if you are infected, as it takes time for enough virus to build up in the body for tests to catch it. So, for those days, I’d just avoid people as much as possible.
After three to four days, PCR tests would start to be effective, and after five to six days, rapid tests would be. So at that point, I’d start testing: using a mix of PCR and rapid tests over the course of several days, up to two weeks after exposure. Studies have shown that the more tests you do, the more likely you are to catch an infection (and this applies to both PCRs and rapids). Daily is the best strategy, but less frequent regimens can still be useful if your access to tests is limited. At the same time, I’d keep track of any new symptoms, as that can be a sign of infection even if all tests are negative.
I’d personally be comfortable hanging out with someone who has had an exposure but consistent negative test results and no symptoms. But others who are less risk-tolerant than I am might avoid any contact for two weeks. The type of contact matters, too: a short, outdoor meeting or one with masks on is safer than a prolonged indoor, no-mask meeting.
Vaccine effectiveness
Another reader asked:
Is there any information on the effectiveness of the latest vaccines, including vaccines that combine Covid and RSV, and are there similarities between these viruses (related?)
As we head into respiratory virus season in the U.S., there will be, for the first time, vaccines available for all three major diseases: COVID-19, the flu, and RSV. I’ll talk about effectiveness for each one separately, because they are all separate vaccines for separate viruses. There’s no combined COVID-RSV vaccine on the market.
COVID-19: We know the fall boosters will target XBB.1.5, a variant that has dominated COVID-19 spread in the U.S. recently. There isn’t much data available on these vaccines yet, because the companies developing them (Pfizer, Moderna, Novavax) have yet to present about their boosters to the FDA and CDC, as is the typical process. The CDC’s vaccine advisory committee is meeting this coming Tuesday to talk fall vaccines, though, so it’s likely we will see some data from that meeting.
Also worth noting: some early laboratory studies suggest that vaccines based on XBB.1.5 will provide good protection against BA.2.86, despite concerns about differences between these variants. (More on this later in today’s issue.)
Flu: Every year, scientists and health officials work together to update flu vaccines based on the influenza strains that are circulating around the world. Effectiveness can vary from year to year, depending on how well the shots match circulating strains.
This week, we got a promising update about the 2023 flu vaccines: CDC scientists and colleagues studied how well these shots worked in the Southern Hemisphere, which has its flu season before the Northern Hemisphere. The vaccine reduced patients’ risk of flu-related hospitalization by 52%, based on data from several South American countries that participate in flu surveillance. This is pretty good by flu vaccine standards; see more context about the study in this article from TIME.
RSV: There are two new RSV vaccines that will be available this fall, both authorized by the FDA and CDC in recent months. These vaccines—one produced by Pfizer, one by GSK—both did well in clinical trials, reducing participants’ risks of severe RSV symptoms by about 90% (for the first year after infection, with effectiveness declining over time).
Both vaccines were authorized specifically for older adults, and Pfizer’s was also authorized for pregnant people as a protective measure for their newborns. We’ll get more data about these vaccines as the respiratory virus season progresses, but for now, experts are recommending that eligible adults do get the shots. This article from Yale Medicine goes into more details.
Nasal sprays as COVID-19 protection
Another reader asked:
I’m thinking of researching what foods and supplement are anti-viral anti-COVID. I’m wondering if anyone has done any research on that?
I haven’t seen too much research on about foods and supplements, since dietary options are usually not considered medical products for study. Generally, having a healthy diet can be considered helpful for reducing risk from many health conditions, but it’s not something to rely on as a precaution in the same way as you might rely on masking or cleaning air.
Another thing you might try, though, would be nasal sprays to boost the immune system. I have yet to try these myself, but have seen them recommended on COVID-19 Safety Twitter and by cautious friends. The basic idea of these nasal sprays is to kill viruses in one’s upper respiratory tract, essentially blocking any coronavirus that might be present from spreading further. People take these sprays as a preventative measure before potential exposures.
A couple of references on nasal sprays:
- Does nitric oxide nasal spray (Enovid/VirX/FabiSpray) help prevent or treat COVID-19? (Those Nerdy Girls)
- As COVID market narrows, SaNOtize moves to carve a new one: over-the-counter prevention (Fierce Biotech)
- Clinical efficacy of nitric oxide nasal spray (NONS) for the treatment of mild COVID-19 infection (Scientific paper in The Journal of Infection)
-
Updated COVID-19 vaccines are coming this fall
This past Thursday, the FDA’s advisory committee on vaccines and similar biological products met to discuss COVID-19 boosters for this fall. They voted in favor of updating the vaccines based on Omicron XBB, a variant lineage that has dominated both in the U.S. and globally this year.
Here are a few key points from the meeting, citing from Your Local Epidemiologist and the Associated Press’ coverage:
- The fall boosters will be monovalent, meaning they’ll only include the XBB strain—unlike our most recent boosters, which were bivalent (including BA.4/BA.5 and the original, Wuhan strain). The FDA has recommended this switch because research suggests monovalent vaccines may be more effective, and because the original coronavirus strain is no longer circulating; we’re mostly seeing XBB right now.
- The FDA has not yet decided which exact variant will be used for this fall’s boosters. While experts generally agree that it should be an XBB lineage, the FDA will make a final call on this closer to the fall respiratory virus season. XBB.1.5, XBB.1.9, and XBB.1.16 are all major contenders right now.
- This fall’s vaccination campaign is likely to prioritize at-risk populations, including seniors and those with medical conditions that damage their immune systems, similar to the bivalent booster shot rollouts. Ongoing vaccine effectiveness research suggests that these groups benefit most from the protection of an additional booster shot, though people not in these groups obviously benefit as well.
- The CDC will make final decisions about which groups will most need the fall boosters, as well as whether some groups may be eligible for more than one of the shots. Children may also become eligible for new boosters; that’ll be up to the CDC as well.
- In choosing XBB for the fall boosters, the FDA is standardizing with recommendations from the World Health Organization and European Union, which have also suggested that XBB be the target for the next boosters. Last year, the WHO recommended BA.1, while the U.S. used BA.4/BA.5. Standardizing will be helpful for ongoing data collection, since…
- Data problems persist: I’ve written a lot about the U.S.’s disadvantages in tracking vaccine effectiveness, particularly compared to other countries with more standardized health systems. This problem has persisted through all rounds of boosters, including the shots planned for this fall; in fact, it’s even harder now for U.S. agencies to monitor how well the vacines work, as the federal public health emergency’s end led to fewer data collection authorities for the CDC. (Safety monitoring systems will continue, though.)
It’s also worth noting that the boosters this fall will be the first major COVID-19 vaccine rollout following the end of the federal public health emergency. While the Biden administration has devoted some funding for getting vaccines to uninsured Americans, most people will now be getting vaccinated through their health insurance.
This is certain to make the process more complicated and more challenging for many. I’ve already seen stories of people who are eligible for a second bivalent booster having a hard time getting that shot. (See this recent Death Panel episode, for example.) The federal government is doing very little to improve this situation in time for the fall boosters to arrive—and no matter how well XBB vaccines work in theory, they’ll do little in practice if nobody can actually get them.
-
Sources and updates, April 16
- Long COVID care access challenges: A new paper, published this week in JAMA Network Open, shares the results of a survey by the Urban Institute think tank. The researchers surveyed about 9,500 adults, including 800 with self-reported Long COVID, about their experiences accessing medical care. The long-haulers were more likely to report difficulties with accessing and paying for care, compared to adults who don’t have the condition. To address this issue, the healthcare system needs to develop clinical guidelines for Long COVID, train workers about it, address insurance barriers, and more, the researchers said.
- PolyBio announces Long COVID research agenda: Speaking of Long COVID: the PolyBio Research Foundation, a nonprofit devoted to Long COVID, ME/CFS, and other chronic conditions, has announced several research projects that it’s supporting. The projects will evaluate potential biological mechanisms underlying Long COVID symptoms, such as virus persisting in different parts of the body, changes in T cell activity, microclots, and more. PolyBio has a great reputation for pushing ahead post-viral disease research, and I’m looking forward to seeing the results of these studies.
- Bivalent boosters hold up against XBB variants: Another new study that caught my attention this week: researchers at the University of North Carolina and North Carolina state health department reported on how well the bivalent, Omicron-specific boosters worked, based on the agency’s surveillance data. The study examined data from September 2022 through February 2023, a period when the BQ and XBB subvariants were dominating coronavirus spread. North Carolina residents who received the bivalent boosters were significantly less likely to experience severe COVID-19 symptoms, the researchers found, but their protection started to wane within a month after receiving the shots.
- Resources on indoor air quality in schools: Journalist’s Resource recently updated this list of research and resources for journalists interested in covering indoor air quality in K-12 schools. The update follows a CDC report showing that many public schools across the U.S. have failed to upgrade their ventilation, despite federal funding to do so (which I covered last week). School air quality is a topic that deserves more reporting, especially from local journalists who can dig into how their school districts are doing.
- Arizona county starts monitoring for a fungus in wastewater: I’m always on the lookout for new uses of wastewater surveillance, and one promising application could be tracking Candida auris, a fungal pathogen that’s resistant to common drugs and spreads quickly in healthcare settings. The Arizona state health department and a lab at the University of Arizona recently launched a pilot program to track this fungus through Yuma County’s wastewater. Arizona and neighboring southwest states have been a hotbed for C. auris; if this pilot is successful, other states could start similar efforts.
-
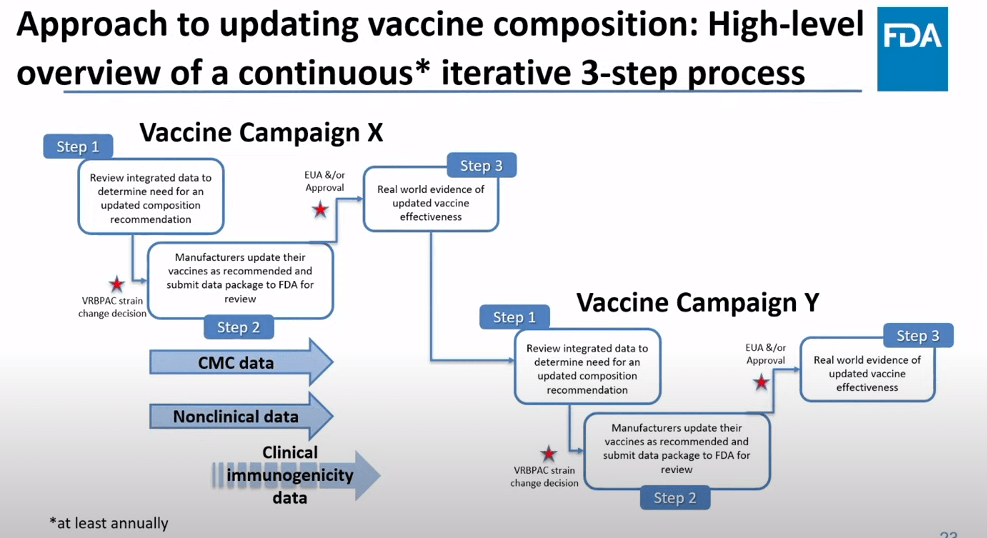
The future of COVID-19 vaccines needs more data
The FDA recommends that the U.S. shifts to annual COVID-19 vaccines, with a variety of data sources feeding into decision-making. Screenshot from the VRBPAC meeting on January 26, 2023. On Thursday, the FDA’s Vaccines and Related Biological Products Advisory Committee (or VRBPAC) met to discuss the future of COVID-19 vaccines. While the committee readily agreed that our current, Omicron-specific shots are working well and should be used more broadly, it had a hard time answering other questions about future vaccine regimens—largely due to a lack of good data.
Now, the lack of good U.S. data on vaccine effectiveness is not a new problem. I personally have been writing about this since fall 2021, to the point that I feel like a broken record for bringing it up again. To summarize: the U.S. has a fractured health system in which every state tracks vaccinations differently, with a lot of local public health departments and private companies in the mix, too. As a result, it’s challenging for researchers to determine exactly who is getting COVID-19 after vaccination and how the virus is impacting them.
This lack of detailed vaccine effectiveness data was a problem in fall 2021, when federal officials decided on an initial round of booster shots. And it’s still a problem in winter 2023, as the same officials attempt to plot out a future in which COVID-19 is another disease that we deal with on an annual basis.
But this week’s VRBPAC meeting revealed some other areas of data that are also lacking as we try to answer questions about future vaccines. Here’s my summary of five primary data gaps that came up at the meeting, and some suggestions for potential solutions.
Detailed vaccine effectiveness data
The biggest data gap, of course, is our lack of answers to the question: Who is getting sick with COVID-19 after vaccination? And related questions: How sick did they get? Which variants did they get sick with? What preexisting conditions or comorbidities did they have?
Our lack of standardized medical data in the U.S. makes it tough to answer these questions at the population level. Analyzing variants is particularly tricky, given that variant surveillance in the U.S. tends to be entirely anonymized—with no connections between the genomic sequencing of random PCR tests and the health outcomes (or vaccination statuses) of those patients. And analyzing preexisting conditions can be crucial as officials try to decide which groups of people need extra boosters, but these conditions often are not collected in standard databases or linked to COVID-19 records.
As a result, U.S. officials tend to rely on other countries with more comprehensive, standardized data systems for information on how well the vaccines work. We also have to rely on the pharmaceutical companies producing these vaccines, which often don’t openly share their data—they tend to present clinical trial results in press releases, over peer-reviewed studies. Companies also tend to do trials that align better with their own financial interests, rather than looking at the full scope of vaccine effectiveness.
Even in this week’s VRBPAC meeting, scientists from Moderna presented results from a clinical trial—conducted in the U.K.—that tested the company’s bivalent boosters against the original (non-Omicron) boosters.
Better tracking of variants
The primary reason why our COVID-19 vaccines require updates in the first place is the coronavirus’ continued evolution. Every new lineage of Omicron that rises to prevalence is either a bit better at spreading quickly, a bit better at evading immunity from prior infection or vaccination, or both. To successfully tweak our vaccines in the future, scientists will need to know which variants are out there and how dangerous they are.
Right now, variant tracking largely relies on PCR testing, as researchers randomly select some swab samples to sequence. But with fewer and fewer people getting PCR tests, the sample pool is dwindling. As a result, to stay ahead of new variants, the U.S. needs to diversify its surveillance options. That will likely include more variant sequencing from wastewater (as a population-level COVID-19 sample), more sequencing at hospitals and healthcare centers, and more travel surveillance focused on international variant patterns.
Variant surveillance will also need to inform how suited U.S.-developed COVID-19 vaccines are for the rest of the world. Right now, the pharmaceutical companies that have produced the most effective vaccines (i.e. Pfizer and Moderna) are American—so American regulators are essentially dictating vaccine policy for the world, even though their priority is the U.S. FDA official Jerry Weir said as much at the meeting. U.S. hegemony over COVID-19 vaccines will continue to be a complex, fraught topic going forward.
Tracking different types of immunity
At the VRBPAC meeting, Moderna, Pfizer, and Novavax all presented data on how well their vaccines work against currently-dominant coronavirus variants. While they included some clinical data (case rates, hospitalization rates), the presentations mostly focused on one metric: antibody titers. To calculate if a vaccine works against a certain variant, the easiest strategy is measuring the antibodies produced after a vaccinated blood sample is exposed to that variant.
While this is the easiest strategy, it’s far from the only way to examine how well a vaccine works. Members of the VRBPAC committee frequently asked the pharmaceutical companies for those other metrics: T cells, B cells, and more ways of measuring the immune system’s response to COVID-19. But the companies had little response to these questions. Even FDA and NIH officials at the meeting admitted that they still didn’t have a good understanding of how, exactly, our current vaccines impact our immune systems, beyond generating antibodies.
To better evaluate future vaccines, scientists will need to get better at measuring other aspects of our immune responses. That includes future mRNA vaccines as well as next-generation vaccines in the works right now, such as nasal vaccines (recently authorized in China and India) and vaccines designed to protect against all variants (currently in development at Duke University and other institutions).
I also think it’s worth noting that, as Katelyn Jetelina writes in her coverage of the VRBPAC meeting at Your Local Epidemiologist, the FDA could require pharmaceutical companies to study the immune system more holistically when they submit further vaccine updates for authorization. “The FDA could require sponsors to do detailed investigations, e.g. assessing lymph nodes, bone marrow, and breakthroughs,” she writes. “This would help us understand immunity better, so we can make better recommendations. It’s not clear why they aren’t pushing for this.”
Improving vaccine safety tracking
Two years after the first COVID-19 vaccines were authorized, we now know that the vaccines are overwhelmingly safe and effective. Most people have mild side effects following their shots, like sore arms and fatigue, but the benefits of getting vaccinated far outweigh the risks. However, some discussion at the VRBPAC meeting indicated that federal agencies could do a better job of tracking rare (yet important) serious side effects.
For example, a safety presentation from the Kaiser Permanente Vaccine Study Center suggested that there might be a small increase in stroke risk for older adults who get vaccinated. The risk has only appeared in one vaccine safety database so far and appears to be minimal, per the FDA, but it’s still worth closer examination.
In addition, as Helen Branswell and Matthew Herper discuss in the STAT News liveblog, the VRBPAC meeting didn’t present much new data about vaccine safety risks for children, such as myocarditis among boys and young men. Plus, we have limited data so far on whether vaccination may contribute to autoimmune conditions or Long COVID-like symptoms, a problem that has shown up in some studies and anecdotal reports.
If public health officials are going to continue encouraging Americans to get COVID-19 shots once a year (or more), they will need to thoroughly address concerns about these potential side effects. This is particularly true for young children, a group that’s been vaccinated at fairly low numbers so far.
Navigating COVID-19’s interactions with other vaccines
At the VRBPAC meeting, FDA officials suggested a potential future in which most Americans get one COVID-19 vaccine per year, on a similar timeline to the annual flu shot. Variant strains might be selected in the spring or summer, with vaccines developed and produced in time for a fall vaccination campaign. Some at-risk groups (older adults, people with compromised immune systems, etc.) might get two doses each year.
To make this possible, the VRBPAC committee members suggested that we’ll need to track how COVID-19 vaccines intersect with other vaccines. For example, if an older adult receives their flu shot and COVID-19 shot in the same doctor’s visit, does that dampen how well one or the other vaccine works? Does it increase the risks of severe side effects? We don’t know, at this point.
Another major area of future study will be how COVID-19 vaccines may fit into regular, childhood immunization schedules for young kids. Similarly to the COVID-19 plus flu question, scientists will need to track any potential interactions between COVID-19 shots and other regular shots—along with answering questions about how many shots are needed, timing between shots, and more.
One day, I’m sure, we will have a regular COVID-19 vaccination schedule in the U.S. that runs parallel to our flu vaccination schedule. But it will take time, discussions, and a lot more data to get there.
More vaccination data