- CDC warns of risk to immunocompromised people: As of January 2023, there are no longer any monoclonal antibody treatments available for COVID-19, as these treatments do not provide protection against recent versions of Omicron. The no-longer-effective treatments include Evusheld, a drug used as a protective measure (to reduce risk of symptomatic COVID-19 for immunocompromised people. With Evusheld now unavailable, the CDC issued recommendations last week for people who have severely compromised immune systems. Of course, the CDC’s recommendations are largely targeted to individual action; to actually protet this vulnerable group, all Americans would need to follow collective public health measures.
- Modeling COVID-19 as a persistent “endemic“: A recent preprint, from researchers at the drug company Fractal Therapeutics and collaborators, estimates just how challenging it is for people to avoid COVID-19 when the disease is not managed at a societal level. The researchers estimated Americans’ long-term COVID-19 risk based on an epidemiological model incorporating frequent reinfections, and limited individual-level protections. People who are vaccinated but don’t take other measures to reduce their risk of getting COVID-19 “can expect to spend an average of 6 days a year acutely sick with COVID-19 and also incur a 12% risk of long COVID (symptoms lasting more than 3 months),” the researchers write.
- Global COVID-19 vaccination rate: Another new study, published in the CDC’s Morbidity and Mortality Weekly Report, provides an update on global vaccination rates, calculated by researchers at the World Health Organization. According to the WHO team, about 76% of older adults (ages 60 and older) have received a primary series of COVID-19 vaccines. (The study doesn’t report on booster rates, which are likely much lower worldwide.) As the vast majority of COVID-19 deaths worldwide have occurred among this age group, it should be a priority for vaccination, including the primary series and booster doses.
- COVID-19 litigation database: I recently learned about this database of COVID-related legal documents, run by researchers at the University of Trento in Italy. The database aims to publish case documents from around the world reflecting challenges to COVID-19 policies. As of February 4, it includes documents from 1,978 cases, which can be searched by country, year, type of human rights issue, vulnerable group involved, and more.
- Flu vaccine works well this year: A bit of non-COVID good news: this season’s flu vaccine is well-matched to the flu strain currently circulating in North America, according to a recent study from Canadian researchers and public health officials. Receiving a flu shot halved an individual’s risk of a severe flu case that needed medical care, the study found. Flu shots often have an effectiveness below 40%, explained STAT’s Helen Branswell on Twitter, as the vaccines do not always perfectly match up to circulating viral strains. But this year, the shot appears to be working well.
- NYC declares end of mpox epidemic: And one more bit of good news: New York City officials have declared that the city’s epidemic of mpox (formerly called monkeypox) is now over. The city was a hub for mpox transmission last summer and became a center of the U.S. outbreak; but NYC has reported low case numbers since early fall. The federal public health emergency for mpox also recently ended.
Category: Vaccines
-
Sources and updates, February 5
-
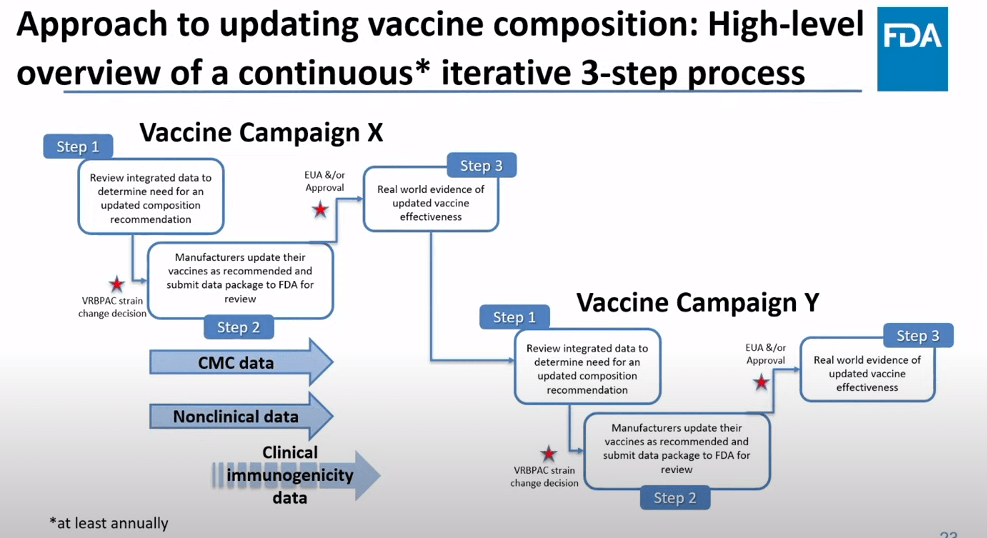
The future of COVID-19 vaccines needs more data
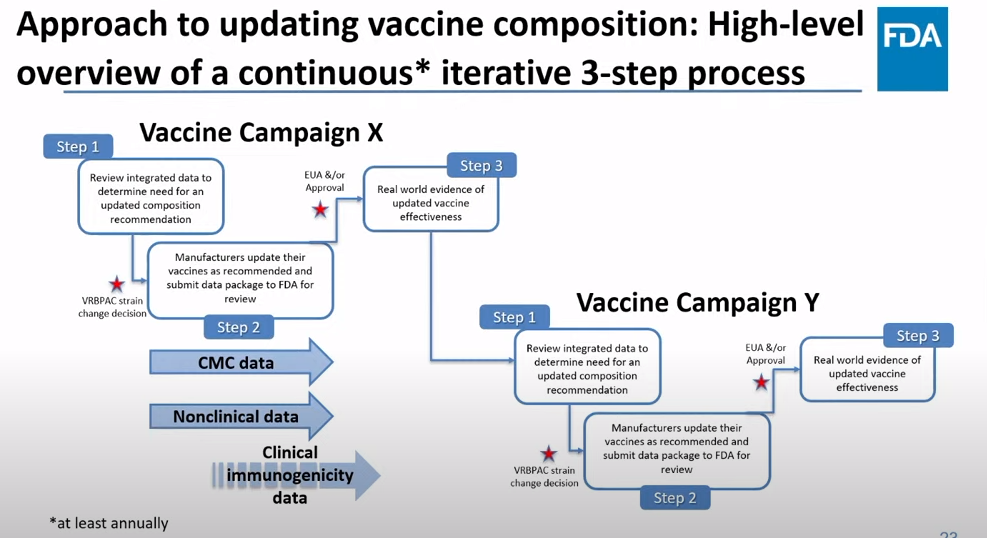
The FDA recommends that the U.S. shifts to annual COVID-19 vaccines, with a variety of data sources feeding into decision-making. Screenshot from the VRBPAC meeting on January 26, 2023. On Thursday, the FDA’s Vaccines and Related Biological Products Advisory Committee (or VRBPAC) met to discuss the future of COVID-19 vaccines. While the committee readily agreed that our current, Omicron-specific shots are working well and should be used more broadly, it had a hard time answering other questions about future vaccine regimens—largely due to a lack of good data.
Now, the lack of good U.S. data on vaccine effectiveness is not a new problem. I personally have been writing about this since fall 2021, to the point that I feel like a broken record for bringing it up again. To summarize: the U.S. has a fractured health system in which every state tracks vaccinations differently, with a lot of local public health departments and private companies in the mix, too. As a result, it’s challenging for researchers to determine exactly who is getting COVID-19 after vaccination and how the virus is impacting them.
This lack of detailed vaccine effectiveness data was a problem in fall 2021, when federal officials decided on an initial round of booster shots. And it’s still a problem in winter 2023, as the same officials attempt to plot out a future in which COVID-19 is another disease that we deal with on an annual basis.
But this week’s VRBPAC meeting revealed some other areas of data that are also lacking as we try to answer questions about future vaccines. Here’s my summary of five primary data gaps that came up at the meeting, and some suggestions for potential solutions.
Detailed vaccine effectiveness data
The biggest data gap, of course, is our lack of answers to the question: Who is getting sick with COVID-19 after vaccination? And related questions: How sick did they get? Which variants did they get sick with? What preexisting conditions or comorbidities did they have?
Our lack of standardized medical data in the U.S. makes it tough to answer these questions at the population level. Analyzing variants is particularly tricky, given that variant surveillance in the U.S. tends to be entirely anonymized—with no connections between the genomic sequencing of random PCR tests and the health outcomes (or vaccination statuses) of those patients. And analyzing preexisting conditions can be crucial as officials try to decide which groups of people need extra boosters, but these conditions often are not collected in standard databases or linked to COVID-19 records.
As a result, U.S. officials tend to rely on other countries with more comprehensive, standardized data systems for information on how well the vaccines work. We also have to rely on the pharmaceutical companies producing these vaccines, which often don’t openly share their data—they tend to present clinical trial results in press releases, over peer-reviewed studies. Companies also tend to do trials that align better with their own financial interests, rather than looking at the full scope of vaccine effectiveness.
Even in this week’s VRBPAC meeting, scientists from Moderna presented results from a clinical trial—conducted in the U.K.—that tested the company’s bivalent boosters against the original (non-Omicron) boosters.
Better tracking of variants
The primary reason why our COVID-19 vaccines require updates in the first place is the coronavirus’ continued evolution. Every new lineage of Omicron that rises to prevalence is either a bit better at spreading quickly, a bit better at evading immunity from prior infection or vaccination, or both. To successfully tweak our vaccines in the future, scientists will need to know which variants are out there and how dangerous they are.
Right now, variant tracking largely relies on PCR testing, as researchers randomly select some swab samples to sequence. But with fewer and fewer people getting PCR tests, the sample pool is dwindling. As a result, to stay ahead of new variants, the U.S. needs to diversify its surveillance options. That will likely include more variant sequencing from wastewater (as a population-level COVID-19 sample), more sequencing at hospitals and healthcare centers, and more travel surveillance focused on international variant patterns.
Variant surveillance will also need to inform how suited U.S.-developed COVID-19 vaccines are for the rest of the world. Right now, the pharmaceutical companies that have produced the most effective vaccines (i.e. Pfizer and Moderna) are American—so American regulators are essentially dictating vaccine policy for the world, even though their priority is the U.S. FDA official Jerry Weir said as much at the meeting. U.S. hegemony over COVID-19 vaccines will continue to be a complex, fraught topic going forward.
Tracking different types of immunity
At the VRBPAC meeting, Moderna, Pfizer, and Novavax all presented data on how well their vaccines work against currently-dominant coronavirus variants. While they included some clinical data (case rates, hospitalization rates), the presentations mostly focused on one metric: antibody titers. To calculate if a vaccine works against a certain variant, the easiest strategy is measuring the antibodies produced after a vaccinated blood sample is exposed to that variant.
While this is the easiest strategy, it’s far from the only way to examine how well a vaccine works. Members of the VRBPAC committee frequently asked the pharmaceutical companies for those other metrics: T cells, B cells, and more ways of measuring the immune system’s response to COVID-19. But the companies had little response to these questions. Even FDA and NIH officials at the meeting admitted that they still didn’t have a good understanding of how, exactly, our current vaccines impact our immune systems, beyond generating antibodies.
To better evaluate future vaccines, scientists will need to get better at measuring other aspects of our immune responses. That includes future mRNA vaccines as well as next-generation vaccines in the works right now, such as nasal vaccines (recently authorized in China and India) and vaccines designed to protect against all variants (currently in development at Duke University and other institutions).
I also think it’s worth noting that, as Katelyn Jetelina writes in her coverage of the VRBPAC meeting at Your Local Epidemiologist, the FDA could require pharmaceutical companies to study the immune system more holistically when they submit further vaccine updates for authorization. “The FDA could require sponsors to do detailed investigations, e.g. assessing lymph nodes, bone marrow, and breakthroughs,” she writes. “This would help us understand immunity better, so we can make better recommendations. It’s not clear why they aren’t pushing for this.”
Improving vaccine safety tracking
Two years after the first COVID-19 vaccines were authorized, we now know that the vaccines are overwhelmingly safe and effective. Most people have mild side effects following their shots, like sore arms and fatigue, but the benefits of getting vaccinated far outweigh the risks. However, some discussion at the VRBPAC meeting indicated that federal agencies could do a better job of tracking rare (yet important) serious side effects.
For example, a safety presentation from the Kaiser Permanente Vaccine Study Center suggested that there might be a small increase in stroke risk for older adults who get vaccinated. The risk has only appeared in one vaccine safety database so far and appears to be minimal, per the FDA, but it’s still worth closer examination.
In addition, as Helen Branswell and Matthew Herper discuss in the STAT News liveblog, the VRBPAC meeting didn’t present much new data about vaccine safety risks for children, such as myocarditis among boys and young men. Plus, we have limited data so far on whether vaccination may contribute to autoimmune conditions or Long COVID-like symptoms, a problem that has shown up in some studies and anecdotal reports.
If public health officials are going to continue encouraging Americans to get COVID-19 shots once a year (or more), they will need to thoroughly address concerns about these potential side effects. This is particularly true for young children, a group that’s been vaccinated at fairly low numbers so far.
Navigating COVID-19’s interactions with other vaccines
At the VRBPAC meeting, FDA officials suggested a potential future in which most Americans get one COVID-19 vaccine per year, on a similar timeline to the annual flu shot. Variant strains might be selected in the spring or summer, with vaccines developed and produced in time for a fall vaccination campaign. Some at-risk groups (older adults, people with compromised immune systems, etc.) might get two doses each year.
To make this possible, the VRBPAC committee members suggested that we’ll need to track how COVID-19 vaccines intersect with other vaccines. For example, if an older adult receives their flu shot and COVID-19 shot in the same doctor’s visit, does that dampen how well one or the other vaccine works? Does it increase the risks of severe side effects? We don’t know, at this point.
Another major area of future study will be how COVID-19 vaccines may fit into regular, childhood immunization schedules for young kids. Similarly to the COVID-19 plus flu question, scientists will need to track any potential interactions between COVID-19 shots and other regular shots—along with answering questions about how many shots are needed, timing between shots, and more.
One day, I’m sure, we will have a regular COVID-19 vaccination schedule in the U.S. that runs parallel to our flu vaccination schedule. But it will take time, discussions, and a lot more data to get there.
More vaccination data
-
Sources and updates, December 18
- Federal government opens up at-home test orders: The Biden administration has revived its program to mail out free COVID-19 at-home rapid tests, just in time for the holidays. Every household can now order four more tests. This feels pretty minimal (and late in the season) for a surge already overwhelming hospitals, but it’s better than nothing. Also, remember to report your results from these tests to the National Institutes of Health’s new portal!
- COVID-19 vaccines saved millions of lives: A new report from the Commonwealth Fund estimates the hospitalizations and deaths saved by two years of COVID-19 vaccines, in honor of the two-year anniversary of those shots first becoming available. About 80% of Americans have received at least one vaccine dose, the authors write, “with the cumulative effect of preventing more than 18 million additional hospitalizations and more than 3 million additional deaths.” The modeling data underlying this analysis are available for download.
- Congressional COVID-19 subcommittee issues final report: House Democrats on the Select Subcommittee on the Coronavirus Crisis recently released their final report, a 200-page document outlining how the U.S. should prepare for the next public health emergency. The report sums up information from three years of research and hearings, including some new findings from more recent investigations. It was released in time with the Subcommittee’s final hearing last Wednesday, which also focused on preparedness. Next year, the Republican-controlled House will have new COVID-19 priorities.
- Helix and CDC build multi-disease surveillance program: This week, leading viral surveillance company Helix announced that its partnership with the CDC has expanded to include sequencing other respiratory viruses, beyond COVID-19. The company will work with major health systems in Minnesota and Washington to track viral variants for the coronavirus, flu, RSV, and other pathogens—and will build infrastructure connecting that sequencing data to electronic health records. That second piece is particularly intriguing, as variant data usually aren’t connected back to health records in the U.S.
- State-level wastewater surveillance expansions: The University of Minnesota is working on a process to test wastewater for the coronavirus, flu, and RSV simultaneously, according to reporting by local outlet KARE11. A team of researchers at the university’s medical school currently test wastewater from 44 sewage treatment plants in Minnesota, and is working to broaden this work with grants from the CDC and state health department. Across the country, New Hampshire’s state health department has announced that it will start publishing results of its COVID-19 wastewater testing program online in the coming weeks. The New Hampshire program includes 14 plants across the state.
-
Sources and updates, December 11
- 2022 America’s Health Rankings released: This week, the United Health Foundation released its 2022 edition of America’s Health Rankings, a comprehensive report providing data for more than 80 different health metrics at national and state levels. The 2022 report includes new metrics tailored to show COVID-related disparities; for example, Black and Hispanic Americans had higher rates of losing friends and family members to COVID-19 compared to other groups. I’ve used data from past iterations of this report in stories before, and I’m looking forward to digging into the 2022 edition.
- FDA authorizes bivalent boosters for young kids: This week, the FDA revised the emergency use authorizations (EUAs) of both Pfizer’s and Moderna’s updated, Omicron-specific booster shots to include children between six months and five years old. Kids who previously got two shots of Moderna’s vaccine for this age group can receive a bivalent booster two months later, while kids who got two shots of Pfizer’s vaccine can receive a bivalent booster as their third dose. (Remember, Pfizer’s vaccine for this age group includes three doses.) The updated EUAs will help protect young children from Omicron infection, though uptake will likely be low.
- CDC updates breakthrough case data: Speaking of the updated boosters: the CDC recently added data on these shots to its analysis of COVID-19 cases and deaths by vaccination status. In September, people who had received a bivalent, Omicron-specific boosters had a 15 times lower risk of dying from COVID-19 compared to unvaccinated people; and in October, bivalent-boosted people had a three times lower risk of testing positive compared to the unvaccinated. The CDC will update these data on a monthly basis.
- Director Walensky discusses authority challenges: One bit of coverage from the Milken Future of Health Summit that caught my attention: CDC Director Dr. Rochelle Walensky talked about the agency’s limitations in collecting data from states, reports Rachel Cohrs at STAT News. Walensky specifically highlighted the challenges that the CDC might face in collecting data when the public health emergency for COVID-19 ends, something I’ve previously covered in this publication.
- Boston establishes neighborhood-level wastewater testing: Finally, one bit of wastewater surveillance news: the city of Boston is setting up 11 new sites to test wastewater, giving local public health officials more granular information about how COVID-19 is spreading in the region. The new initiative is a partnership with Biobot Analytics, the same wastewater testing company that has long worked with Boston, the CDC, and public health institutions across the country. (Boston was one of the first cities to start doing this testing.) Also, speaking of Biobot: the company just added a nice chart of coronavirus variants in U.S. wastewater over time to its dashboard.
-
Sources and updates, December 4
- CDC awards $3 billion to improve public health infrastructure: The CDC announced this week that it has awarded state and local public health agencies a total of $3.2 billion to support hiring and training new workers, along with other infrastructure needs. The agency published a breakdown of all the agencies that received awards, although it has not included specific details on what funds will be used for in each place. Local reporters, if your health department received funding, this might be worth looking into!
- CDC expands wastewater testing for polio: Another notable CDC announcement this week: the agency is expanding its wastewater surveillance for polio to two new areas, Oakland County, Michigan and Philadelphia. Testing wastewater for polio is more complicated than testing it for the coronavirus, as STAT News’ Helen Branswell explains in this article; as a result, the CDC is expanding this surveillance in a more limited capacity than what it’s doing for other viruses, like monkeypox and the flu.
- Majority of COVID-19 deaths are now among vaccinated people: A new report from the Kaiser Family Foundation explains why more than 50% of COVID-19 deaths in the U.S. in recent months were among people who had received at least two vaccine doses. According to KFF, factors driving this trend include the rising share of Americans who are vaccinated, waning protection from initial doses, and low uptake of booster shots—particularly of the Omicron-specific boosters that provide better protection against newer variants. More reason to get the new booster if you haven’t yet!
- Paid sick leave correlates with higher vaccination rates: Speaking of vaccination: a new study from researchers at Drexel University and Boston University found that large U.S. cities with city-wide paid sick leave policies had higher vaccination rates than those without such policies. The correlation was particularly evident in neighborhoods with higher social vulnerability, the researchers found. Expanding paid sick leave could help reduce inequities in vaccination coverage, the paper’s authors recommend.
- No monoclonal antibody drugs are currently authorized in the U.S.: This week, the FDA announced that bebtelovimab, a monoclonal antibody made by Eli Lilly, is no longer authorized for COVID-19 treatments in the U.S. The drug was designed based on older versions of the Omicron variant and doesn’t perform well against BQ.1 and BQ.1.1, the sublineages that are currently causing the majority of new cases in the U.S. As a result, no monoclonal antibodies are currently authorized, though Paxlovid and other treatments are still available.
-
Sources and updates, November 20
- CDC update on COVID-19 mortality trends: This week, the CDC published a detailed report about how deaths from COVID-19 have changed in 2022. Overall, between 2,000 and 4,500 COVID-19 deaths were reported each week between April and September 2022, the CDC researchers found; this is lower than at earlier points in the pandemic, but still represents a loss of more than 100,000 Americans over the course of a year. Older adults and those who were un- or under-vaccinated had a higher risk of death from COVID-19, the researchers found; racial and ethnic disparities have “decreased, but persisted.”
- Moderna reports new data on its bivalent booster: Several studies in the last couple of weeks have indicated that the new, Omicron-specific boosters from Pfizer and Moderna are more effective against new variants than the older vaccines. Moderna provided additional data this week, reporting that its new booster led to five times more antibodies that neutralize Omicron BA.4 and BA.5 compared to earlier booster shots. While Moderna’s study hasn’t yet been peer-reviewed, the results are promising in following a trend from past studies, STAT’s Matthew Herper reports.
- Booster shots could keep kids from missing school: Speaking of the new boosters: a new report from the Commonwealth Fund provides analysis of the boosters’ potential impact on school-aged children, as all kids older than five are eligible for the shots. If 80% of eligible Americans receive their bivalent boosters by the end of 2022, the report suggests, this could save over 46 million days of isolation and over 50,000 hospitalizations for school-aged children, along with other benefits. Even getting kids boosted at the level of flu vaccination in 2020-2021 would prevent millions of days of school from being lost.
- Test to treat is inaccessible to rural Americans: A new study, published this week in JAMA Network Open, examined equity issues with the Biden administration’s Test to Treat initiative. The initiative was designed to provide locations where Americans could get a COVID-19 test and then, if they received a positive result, quickly receive a free antiviral drug. But many people don’t live near available locations, the researchers found: “approximately 15% of the overall US population, 30% of American Indian or Alaskan Native people, and 59% of the rural population lived more than 60 minutes from the nearest site,” they write.
- Perception of local COVID-19 levels: A lot of people are acting with incorrect knowledge of their local COVID-19 risk, a new study in the CDC’s Morbidity and Mortality Weekly Report suggests. Researchers from several medical and public health institutions surveyed people who had recently tested positive for COVID-19 in Detroit, Michigan and DuPage County, Illinois, during June and July, 2022. About half of the 5,000 people surveyed said that they thought local COVID-19 transmission was “low or moderate,” even though it was actually at high levels in both places.
-
Sources and updates, November 13
- Updated booster doses by state: This week, the CDC started reporting how many people have received the bivalent, Omicron-specific boosters by state, including state-level data for several demographic groups (over age 5, over 12, over 18, and over 65). The numbers are low: Vermont and Washington, D.C. have the highest booster rates as of November 9, with 21% and 20% of their populations receiving the bivalent shots, respectively. In about half of states, less than 10% of the population has received an updated booster. (H/t Jason Salemi.)
- Additional data suggests new boosters work against BQs: Speaking of the updated booster shots, a recent preprint from researchers at Emory University, Stanford University, and the NIH found that the new boosters produced several times more neutralizing antibodies against subvariants BQ.1.1 and BA.2.75.2 compared to the older vaccines. This was a small lab study and hasn’t yet been peer-reviewed, but it follows similar evidence from other research suggesting that the new boosters do provide additional protection against the most concerning variants currently circulating in the U.S. (See last week’s post.) If you haven’t gotten a bivalent booster yet, now is a good time!
- More evidence that masks in schools prevent COVID-19 spread: Another notable new study this week, published in the New England Journal of Medicine: a group of researchers from Boston institutions examined the differences in COVID-19 case numbers between public school districts that kept mask requirements in place during spring 2022, and those that lifted their requirements upon a statewide policy change in February. Overall, ending required masking led to “an additional 44.9 COVID-19 cases per 1,000 students and staff” during the remainder of the semester, the researchers found. The study demonstrates that masks are still a useful public health strategy to reduce illness—and risk of Long COVID—in schools.
- Paxlovid may reduce Long COVID risk: When Paxlovid first became available earlier in the year, some Long COVID patients reported that the drug helped alleviate their symptoms. A new study from Ziyad Al-Aly and his team at the Veterans Affairs St. Louis healthcare system provides evidence behind the anecdotal reports, finding that veterans treated with Paxlovid had a 25% lower risk of long-term symptoms, based on their electronic health records. The study has received some criticism (and has not yet been peer-reviewed); to me, it provides motivation for actual clinical trials examining Paxlovid’s use for treating Long COVID. RECOVER is running one such trial, but it won’t start until early 2023.
- Estimating COVID-19 infections from wastewater: And one more study that caught my attention this week: researchers at the University of Florida used a modeling technique called a “mass balance equation” to estimate how many people in Gainseville, Florida were sick with COVID-19 based on the virus’ concentration in wastewater. Using about one year of wastewater data (May 2020 to May 2021), the researchers were able to accurately predict actual infections with an error of just 1%. Translating wastewater data into useful information for public health action has been a major challenge for the growing field, so I was glad to see this study providing a potentially-useful method.
-
Sources and updates, November 6
- New data on Omicron boosters: This week, we got two major updates on the safety and effectiveness of the bivalent, Omicron-specific booster shots from Pfizer and Moderna. First, a study in the CDC’s Morbidity and Mortality Weekly Report examined safety, finding that side effects of the new boosters similar to the side effects of previous vaccines, according to the agency’s vaccine surveillance systems. For example, about 60% of vaccine recipients experienced pain, swelling, or itching in the arms where they received the shot. And second, Pfizer and BioNTech shared new data about the companies’ bivalent booster, suggesting that the new booster produces four times more neutralizing antibodies against BA.4 and BA.5 compared to the original booster shot. The study focused on older adults (over age 55) but is still helpful evidence that the new boosters are more effective against currently-circulating variants.
- NIH RECOVER is preparing its first clinical trial: RECOVER, the National Institutes of Health’s flagship study to understand and eventually treat Long COVID, announced this week that it’s preparing clinical trials to test potential treatments. The first of these trials was recently posted to ClinicalTrials.gov (a site for tracking studies that have received federal funding). This trial will focus on testing Paxlovid for Long COVID patients, and RECOVER anticipates it will begin enrolling patients in early 2023. Patients have previously expressed concerns that RECOVER is moving pretty slowly with trials, considering how many Americans are impacted by Long COVID.
- Patients Rising Now Congressional Scorecard: Speaking of government action on medical issues: Patients Rising Now, an advocacy organization focused on patients with chronic illnesses, recently published its first scorecard for Congressional representatives. The resource grades every Senator and House member in the 117th Congress based on how their voting record aligns with the organization’s priorities. While COVID-19 is not specifically mentioned in the grades, this scorecard could have implications for future pandemic-related votes.
- COVID-19 vaccination and race/ethnicity inequities: A new paper from researchers at the University of Minnesota and Boston University examined how vaccination impacted COVID-19 mortality patterns in Minnesota. During the Delta and Omicron surges, the researchers found, mortality among middle-aged people of color was higher than mortality among white people in an age group ten years older. The paper shows that COVID-19 remains “a pandemic of the disadvantaged,” author Elizabeth Wrigley-Field wrote on Twitter. (Disclaimer: through my work at MuckRock, I am collaborating with BU researcher Andrew Stokes, one of the paper’s coauthors.)
- RSV vaccine(s) could be coming soon: Finally, a bit of good news about another respiratory virus: two potential vaccines for RSV are likely to be under FDA review in the coming months. Pfizer recently reported promising results from a clinical trial of a vaccine for pregnant people, who pass antibodies to their children (thus reducing infant RSV risk). And U.S. pharmaceutical company GSK reported results from a trial testing its RSV vaccine for older adults.
-
Sources and updates, October 30
- More detailed bivalent booster data: As of this week, the CDC is reporting some demographic data for the bivalent, Omicron-specific booster shots. The new data suggest that these boosters have had higher uptake among seniors, with about 11 million people over age 65 receiving a shot (compared to just 60,000 in the 5 to 11 age group). White and Asian Americans have higher booster rates than Black, Hispanic, and Native Americans, suggesting that the new doses are following a similar equity pattern to what we’ve seen with prior vaccines.
- COVID-19 mortality by occupation: A new report by the CDC’s National Vital Statistics System provides a rare area of data we don’t usually get in the U.S.: occupational data. CDC researchers used mortality data from 46 states and New York City to examine risk of death by occupation. People working in protective services, accommodation and food services, and other essential jobs that couldn’t be done remotely had the highest death rates—confirming what many public health experts have suspected throughout the pandemic.
- Life expectancy changes during the pandemic: A new study published in Nature, by researchers at the University of Oxford and other European institutions, estimated how life expectancy changed in 29 countries since the start of the pandemic. After a universal life expectancy decline in 2020, the researchers found, some western European countries “bounced back” in 2021 while the U.S. and eastern European countries did not. The results show the impacts of lower vaccination uptake in the U.S., particularly among younger adults.
- Disparities in Paxlovid prescriptions: Another CDC study that caught my attention this week was this analysis in Morbidity and Mortality Weekly Report (MMWR), describing racial and ethnic disparities in prescriptions of Paxlovid—the antiviral COVID-19 treatment which reduces risk of severe symptoms. Between April and July 2022, the researchers found, the share of COVID-19 patients over age 20 who received a Paxlovid prescription was 36% lower among Black patients than among White patients, and 30% lower among Hispanic patients. More work is needed to make Paxlovid availability more equitable.
- New estimates of Long COVID prevalence: One more notable paper published this week: researchers at Massachusetts General Hospital, Harvard, and collaborators conducted an online survey of about 16,000 U.S. adults who tested positive for COVID-19 in the last two months. Of those survey respondents, 15% reported current symptoms of Long COVID. The survey found that older adults and women were more likely to report Long COVID, while those who were fully vaccinated prior to infection had a somewhat lower risk of long-term symptoms. All of these findings are in line with results from other studies, but it’s helpful to see continued validation of these known trends.
-
Sources and updates, October 23
- Genomic surveillance from international travelers: A new CDC dashboard page provides data from the agency’s program sequencing COVID-19 test samples from people arriving in the U.S. on international flights, aiming to identify and track new variants. This program—a partnership between the agency, Ginkgo Bioworks, and XpresSpa Group—started during the Delta wave in 2021 with flights from India, but has since expanded to include over 1,000 volunteers a week at four major airports. The CDC’s new page reports test positivity for travelers’ samples and variants detected through sequencing.
- Implications of commercializing COVID-19 vaccines, treatments, tests: Researchers at the Kaiser Family Foundation analyzed how the federal government’s decreasing support for key COVID-19 products (vaccines, treatments, and tests) could impact Americans’ access. The government’s supply of these products has been depleted through 2022, and researchers anticipate the national Public Health Emergency will end in early 2023. As a result, Americans will soon likely need to rely on commercial products, leading to major challenges for low-income and uninsured people. (I wrote more about data implications of the PHE ending here.)
- Disparities in flu hospitalizations and vaccinations: Much COVID-19 coverage, including in this publication, has focused on inequitable vaccine uptake. In early 2021, more white Americans were getting vaccinated than minority groups, potentially contributing to higher rates of severe disease in those groups through the second year of the pandemic. A new CDC study in the agency’s Morbidity and Mortality Weekly Report (MMWR) finds that a similar trend has occurred for flu over the last ten years: Black, Hispanic, and Native Americans had lower flu vaccine coverage than white Americans from 2009-10 through 2021-22 seasons, and the same groups had higher flu hospitalization rates. The study suggests equitable vaccination is a problem that goes beyond the pandemic.
- Vaccine coverage among healthcare workers: Another CDC MMWR study that caught my attention this week provides results from a survey of healthcare workers, conducted in spring 2022. Among about 3,700 workers who responded to the survey, about four in five reported receiving a flu shot and two in three reported receiving a COVID-19 booster (during the 2021-22 flu season). Workers with vaccine mandates at their jobs had higher coverage than these averages, while long-term care workers had lower coverage. The results indicate more effort is needed to protect healthcare workers and their patients.
- HospitalFinances.org is revamped, newly available: In 2018, the Association of Health Care Journalists (AHCJ) first launched HospitalFinances.org, a database of financial information on nonprofit hospitals pulling from 990 tax forms. The site has been offline for the past year due to a hosting issue, but is now back thanks to researchers at the University of Missouri (which hosts AHCJ). While this resource isn’t specifically COVID-related, it could be useful to reporters investigating hospitals in their areas.